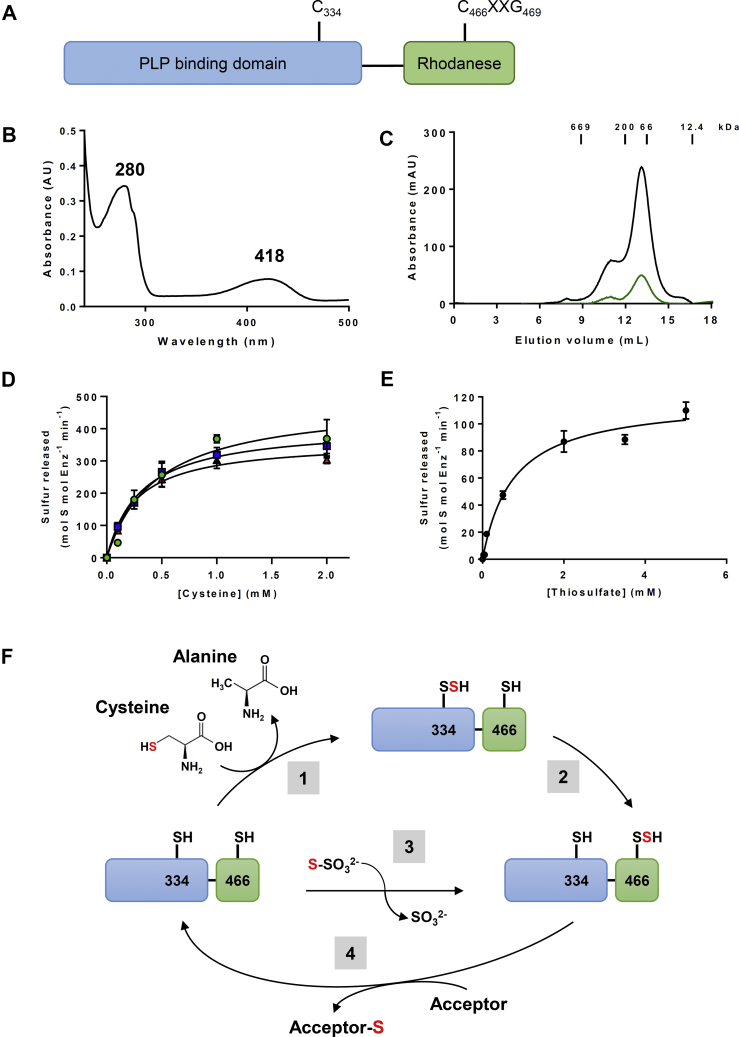
Figure 1.
Pseudorhodoferax CD–Rhd fusion has a dual activity profile.A, modular organization of the Pseudorhodoferax CD–Rhd fusion (WP_056898193.1) presenting the position of the presumed catalytic cysteines of both CD and Rhd domains. B, UV–visible absorption spectrum of the purified N-terminal His-tagged recombinant CD–Rhd recorded in a 30 mM Tris–HCl (pH 8.0) buffer. C, analytical gel filtration (Superdex S200 10/300 column; GE Healthcare) of His-tagged recombinant CD–Rhd (100 μg). The presence of the polypeptide and PLP cofactor has been detected by measuring the absorbance at 280 nm (dark line) and 418 nm (green line), respectively. The apparent molecular weight of CD–Rhd was estimated from the separation of the indicated standards. D, steady-state kinetic parameters of the CD activity. Reactions were performed in the presence of 10 nM CD–Rhd, increasing concentrations of l-cysteine (0–2 mM), and in the presence of various reductants, either 5 mM of DTT (blue squares), or 5 mM GSH (green circles), or 5 mM β-mercaptoethanol (red triangles). The data are represented as mean ± SD of three independent experiments. E, steady-state kinetic parameters of the thiosulfate sulfurtransferase activity. Reactions were performed in the presence of 100 nM CD–Rhd, increasing concentrations of thiosulfate (0–5 mM), and 5 mM β-mercaptoethanol. The data are represented as mean ± SD of three independent experiments. F, proposed mechanism for the CD and sulfurtransferase activities of the CD–Rhd fusion. For the CD activity, the catalytic cysteine (Cys334) of the CD domain (in blue) catalyzes the cysteine desulfuration, leading to its persulfidation and the concomitant release of alanine (1). Then, the sulfur atom is transferred to the catalytic cysteine (Cys466) of the Rhd domain (in green) through a transpersulfidation reaction (2). Cys466 is also responsible for thiosulfate sulfurtransferase activity through the conversion of thiosulfate into sulfite (3). Under persulfidated form, Rhd domain promotes sulfur transfer to acceptors leading to the reduction of CD–Rhd (4). CD, cysteine desulfurase; PLP, pyridoxal 5′-phosphate; Rhd, rhodanese.