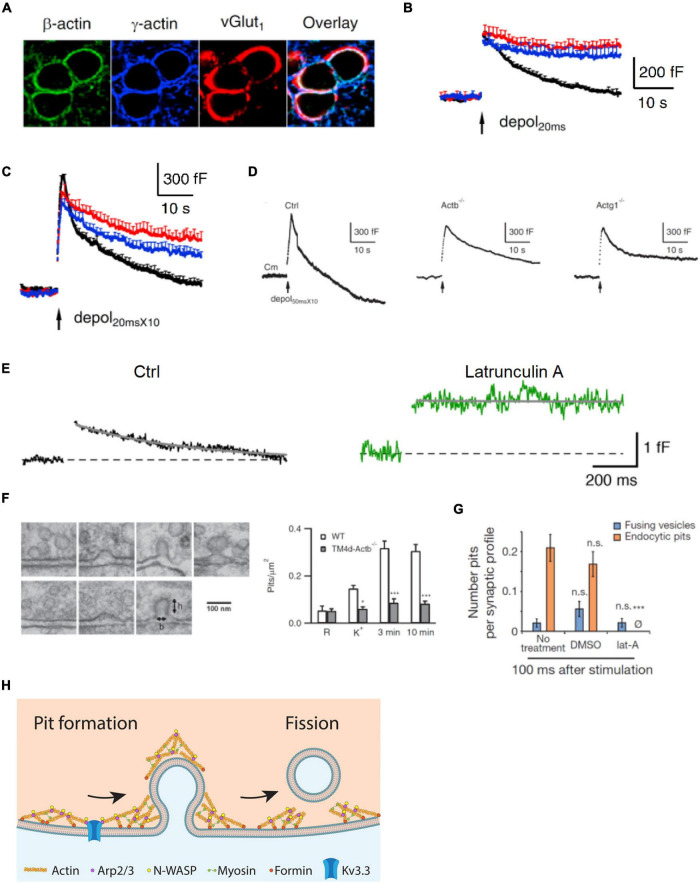
FIGURE 1.
Actin is involved in mediating ultrafast, fast, slow, bulk, and overshoot endocytosis at synapses likely by facilitating membrane pit formation. (A) Antibody staining of β-actin, γ-actin, and vesicular glutamate transporter 1 (vGluT1) in calyx of Held nerve terminals. (B) Actin involvement in slow endocytosis: mean capacitance (Cm) traces (mean + SEM) induced by a 20 ms depolarization from –80 to +10 mV (depol20 ms, arrow) in calyces of wild-type (black), Actb–/– (red), and Actg1–/– (blue) mice. Depol20 ms induces slow endocytosis in wild-type calyces. (C) Actin involvement in rapid (or fast) endocytosis: similar arrangement as in B except that the stimulus was 10 depol20 ms at 10 Hz (depol20 msX10), which induces rapid (or fast) endocytosis in wild-type calyces. (D) Actin involvement in bulk endocytosis and endocytosis overshoot: sampled Cm induced by depol50 msX10 (10 depol50 ms at 10 Hz) with 5.5 mM calcium in the bath from wild-type (Ctrl), β-actin (Actb)–/–, and γ-actin (Actg1)–/– calyces. Depol50 msX10 induces bulk endocytosis (a large step of downward capacitance shift) and endocytosis overshoot in Ctrl. (E) Actin involvement in very fast endocytosis: averaged Cm response to single action potentials in Ctrl (black) and in the presence of latrunculin A (Lat A, green). Gray solid lines are exponential fits to the Cm decay. (F) Left: electron microscopy images of membrane pits of various shapes obtained during or after high potassium chloride (KCl) application from either wild-type (WT) or Actb–/– hippocampal cultures. Right: the number of pits before (R) and after KCl application (K+, 0 min; 3 min and 10 min) in wild-type (WT) control and Actb–/– hippocampal synapses (mean + SEM). *p < 0.05; ***p < 0.001 (t-test). The data show that β-actin knockout inhibits pit formation. (G) Actin involvement in ultrafast pit formation: average number of exocytic pits (blue) and endocytic pits (orange) in cells treated with latrunculin A (Lat A) or dimethyl sulfoxide (DMSO). ***p < 0.001 (t-test). (H) Schematic diagram showing the involvement of F-actin and its nucleation factors, such as Kv3.3 potassium channel, Arp2/3, formin, and myosin II in all kinetically distinguishable forms of endocytosis, including ultrafast, fast, slow, bulk, and overshoot endocytosis. Panels A–D,F are adapted from Wu et al. (2016) with permission. Panel E is adapted from Delvendahl et al. (2016) with permission. Panel G is adapted from Watanabe et al. (2013) with permission.