Abstract
Technological revolutions in several fields have pushed the boundaries of vaccine design and provided new avenues for vaccine development. Next-generation vaccine platforms have shown promise in targeting challenging antigens, for which traditional approaches have been ineffective. With advances in protein engineering, structural biology, computational biology and immunology, the structural vaccinology approach, which uses protein structure information to develop immunogens, holds promise for future vaccine design. In this review, we highlight various vaccine development strategies, along with their advantages and limitations. We discuss the rational vaccine design approach, which focuses on structure-based vaccine design. Finally, we discuss antigen engineering using the epitope-scaffold approach, gaps in structural vaccinology, and remaining challenges in vaccine design.
Keywords: vaccine, immunization, rational design, epitope-scaffolding, epitope-mapping, structural vaccinology, immunogen design, immune focusing, protein engineering
Graphical abstract
1. Introduction
In the past few decades, our understanding of immunology and disease has improved dramatically due to the molecular characterization of pathogen-mediated mechanisms of immune evasion. Technological advances have contributed immensely to the development of new vaccines that have been used successfully around the world to contain many diseases. Vaccines are biological products used to induce a safe immunological response to protect against infection [1]. Ideally, vaccines are medications that can be administered safely to generate long-lasting prophylactic and therapeutic immunity [2]. On a global scale, vaccines have proven successful in reducing the mortality and morbidity caused by many infectious diseases. It is estimated that vaccines prevent 2.5 million deaths worldwide each year, or about 7,000 deaths per day [3].
A successful vaccine is highly effective; it effectively protects the vaccinated patient from disease [4]. Epitopes that induce an effective response against pathogen are called protective epitopes, and these protective epitopes are normally selected in the formulation of a vaccine. Current vaccine development uses state-of-the-art, innovative technologies. Vaccines are one of the most essential tools for maintaining the medical well-being of present and future human generations. Traditional approaches to vaccine development have resulted in an extensive catalog of human vaccines. However, in the setting of challenging pathogens such as antigenically variable viruses, these traditional approaches have not produced effective vaccines [5–7]. Traditional approaches are not fast enough to respond to emerging virus outbreaks. Other important considerations in vaccine development include large-scale production capacity and vaccine stability for transportation and storage purposes. Therefore, advanced vaccine platforms are required to overcome current limitations and develop vaccines that are robust, safe, and inexpensive to manufacture. With recent advances in structural biology, computational biology and immunology, rational vaccine design has become a highly active research field.
Structure-based vaccine design, also known as structural vaccinology, is one such rational vaccine design approach in which immunogens are rationally engineered using available structural information [8–10]. Structural vaccinology promises new vaccines against traditionally difficult targets. Using structural vaccinology, methods have been developed to obtain immunogens in simplified form with well-defined epitopes, known to induce specific neutralizing antibodies. Using this methodology, it is also possible to modify the antigenic proteins, either to enhance antigenicity or to mask the non-neutralizing epitopes in order to avoid the induction of off-target antibodies. One such modification is locking the antigen into a certain conformation for optimal epitope presentation. The structural vaccinology approach can improve the thermostability of vaccines and potentially solve transportation and storage problems. Structural vaccinology, along with a better understanding of immune responses to vaccines, will advance the next generation of rationally designed vaccines.
Here, we focus on some existing approaches to vaccine development and their advantages and limitations. We discuss how the structural vaccinology approach can be used to increase the efficacy of vaccines. We also highlight the critical steps in the structural vaccinology approach. We further review recent epitope-scaffold efforts on engineering immunogens.
2. Vaccine development strategies
Traditional vaccination relies on two specific types of microbiological preparations to develop vaccines. These two categories are: (1) living microbes in a weakened state [11] and (2) inactivated / killed microbial preparations [12]. However, advances in vaccinology have provided alternative methods of developing safer and improved vaccines using various platforms, such as subunit vaccines, toxoids, recombinant vaccines, peptide-conjugated subunit vaccines, and nucleic acid vaccines (Fig. 1).
Figure 1.
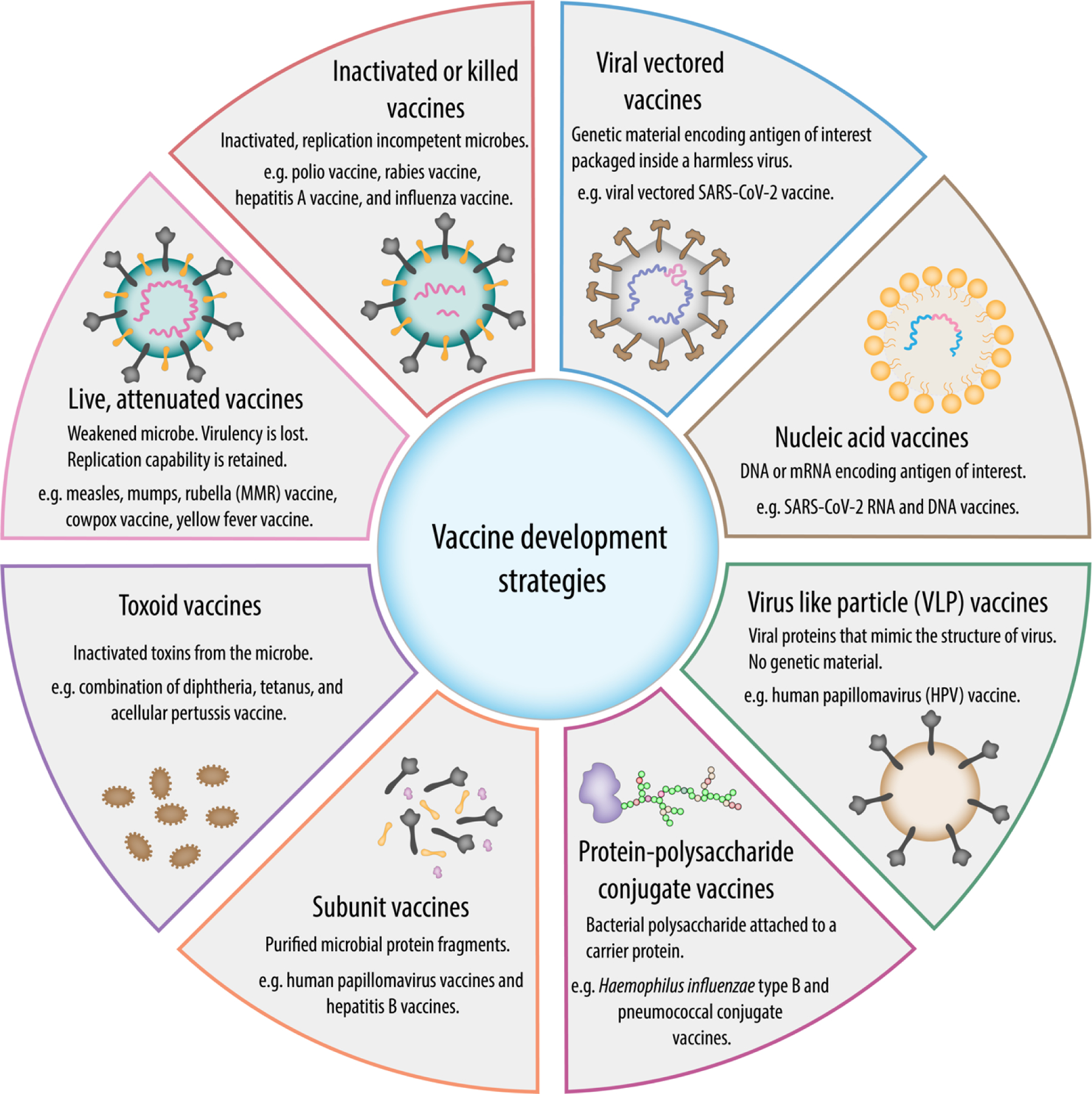
Schematic representation of different approaches used for vaccine development.
2.1. Live, attenuated vaccines
Live attenuated vaccines induce strong immunologic responses and are highly effective. Typically, live vaccines are produced by deleting virulent genes while preserving replication capability [13,14]. Attenuated vaccines can produce strong cell-mediated and humoral responses [15]. Because a viable, replicating pathogen is used, these organisms replicate sufficiently in an immunocompetent host to elicit a strong immune response, but they do not cause significant disease manifestations. Since replication is maintained by the attenuation process, a single dose of the vaccine is usually sufficient to induce long-lasting immunity. In addition, only a small amount of the vaccine needs to be administered to the patient, which maximizes cost-effectiveness. The potential reversion to virulence is one of the critical risk factors associated with live attenuated vaccines [15]. Therefore, they are not suitable for individuals with weakened immune systems. Examples of live, attenuated vaccines against viral infections include the measles, mumps, rubella (MMR) vaccine, cowpox vaccine, yellow fever vaccine, and oral polio vaccine; examples of live, attenuated bacterial vaccines include BCG and tuberculosis.
2.2. Inactivated or killed vaccines
Inactivated or killed vaccines do not contain live pathogenic organism. These vaccines consist of preparations of replication incompetent microorganisms that are either killed or inactivated by various methods such as heat-inactivation, chemical inactivation or radiation, while preserving the structural integrity of the surface antigenic epitopes [13,16,17]. Killed vaccines mainly induce systemic humoral responses with neutralizing antibodies. These vaccines can be given immunocompromised individuals because the dead microorganisms cannot revert back to pathogenic state. However, inadequate inactivation can leave the microbe in its pathogenetic state, whereas excessive inactivation treatment can completely inhibit immunogenicity. The presence of additional excessive surface antigenic sites may cause severe allergic reactions [13]. Usually, inactivated vaccines provide shorter-lasting immunity compared to live vaccines. Therefore, multiple doses may be required over time to boost the immune system. Examples of inactivated vaccines against viral infections include the injected form of polio vaccine, rabies vaccine, hepatitis A vaccine, and the influenza vaccine.
2.3. Toxoid vaccines
Some bacteria can cause disease by secreting toxins. Toxoid vaccines are made using inactivated exotoxins produced by such bacteria. The purified exotoxins are inactivated and toxicity is suppressed by heat, chemicals, or other treatments without altering the immunogenic sites [13,14,18]. The immune system recognizes and eliminates these toxins and provides immunity to the disease. This method is limited to diseases where the bacterial toxins are the main cause of the disease. An example of a toxoid vaccine is the combination of diphtheria, tetanus, and acellular pertussis vaccine.
2.4. Subunit vaccines
Instead of using the entire microbe for immunization, subunit vaccines contain only a portion of the microbe (protein, polysaccharides or a combination of both). Microbial content is purified and used to produce a protective immune response [13,14,19]. Subunit vaccines typically contain 1 to 20 antigens. Since there are no live pathogens in subunit vaccines, there is no risk of virulence reversal or serious side effects. With the advances in biotechnology, it is possible to identify the major antigenic components of microbes. Instead of purifying from the microbes, these antigenic components can be produced using recombinant DNA technology. Vaccines produced using this method are called ‘recombinant subunit vaccines’. Sometimes the production of a recombinant vaccine protein in an organism can alter the conformation of the epitope. As a result, vaccine protein may not be able to induce neutralizing antibodies. Examples of subunit vaccines include human papillomavirus vaccines and hepatitis B vaccines.
2.5. Protein-polysaccharide conjugate vaccines
These vaccines are made by covalently attaching a bacterial surface polysaccharide to a protein. This protein-polysaccharide conjugation produces a long-lasting protective immunity compared to the polysaccharide alone [20]. The conjugating protein can either be a related protein antigen from the same pathogenic microbe that enhances the specific immune response to that pathogen, or an immunogenic protein that serves as an adjuvant or stimulant of the general immune response. The diphtheria and tetanus toxoids are widely used as carriers in conjugate vaccines. A modified version of diphtheria toxoid known as CRM197 (cross-reacting material 197) has been shown to be safe and effective in humans [4]. Examples of conjugated vaccines include Haemophilus influenzae type B and pneumococcal conjugate vaccines.
2.6. Virus like particle (VLP) vaccines
VLPs are virus-derived, multiprotein structures with the ability to self-assemble and mimic the form and size of a native virus particle [6,21,22]. VLPs are formed through the spontaneous interaction between viral structural capsid proteins to form the final structure having geometrical symmetry. VLPs are divided into different categories based on their structural complexity. VLPs lack the viral genome, hence, they are not capable of infecting the host cell, yielding potentially safer vaccine candidates. VLPs offer the effectiveness of a live-attenuated vaccine because of their highly repetitive display of antigenic surface epitopes. VLPs are capable of stimulating both humoral and cellular immune responses [21]. Even in the absence of adjuvants, VLPs generate strong immune responses [23]. To provoke more effective immune responses, VLPs can also be loaded with immune-modulators. Examples of VLP vaccines are the human papillomavirus (HPV) vaccine and the hepatitis B vaccine.
2.7. Nucleic acid vaccines
Nucleic acid-based vaccines (DNA and RNA vaccines) are relatively new vaccine platforms created by manipulating nucleic acids to produce multiple copies of viral antigenic target proteins upon immunization. Nucleic acid vaccines have received special attention because of their potential to rapidly manufacture vaccines against emerging infectious diseases such as COVID-19. Nucleic acid vaccines generate endogenous viral proteins having native conformation and posttranslational modifications. Nucleic acid vaccines have been shown to induce both humoral and cell mediated responses [6]. Simplicity of the vector, uncomplicated delivery, and duration of expression are some of the most important advantages of nucleic acid vaccines [24].
DNA vaccines are the preparations of purified plasmids that contain one or more desired DNA sequences that are capable of inducing an immune response against a pathogen [25]. Once the plasmid DNA preparations are delivered by a variety of routes, including intramuscular (IM), intradermal (ID), mucosal, or transdermal routes, the host cells take up the introduced DNA, resulting in the expression of the gene to produce appropriate proteins within the cell [26]. Proteins are processed by the host proteases into small antigenic peptides and presented on the cell surface of antigen presenting cells (APCs). DNA vaccines can induce both humoral and cellular immunity and are safe, temperature stable and relatively simple [26]. DNA can be synthesized by simple scalable chemistry or produced on a large scale in bacteria, hence DNA vaccines are much cheaper than protein, whole-cell, or viral vaccines [27]. To improve the immunogenicity, plasmids can be constructed to include multiple antigens. The main disadvantage of DNA vaccines is their low immunogenicity due to the low rate of transfection and subsequent protein expression.
On the other hand, mRNA vaccines are the simplest nucleic acid vaccines and a promising platform for vaccine development. mRNA vaccines work by delivering antigen-encoding mRNA directly to APCs in vivo [28]. mRNAs can be efficiently introduced into APCs via multiple pathways. As soon as the mRNAs have been released in the cell, they are translated into corresponding antigenic proteins in the cytoplasm. Antigen proteins are then processed into peptides and after binding the major histocompatibility complexes (MHCs), presented on the cell surface [28]. There are currently non-amplifying and self-amplifying mRNA vaccines that are categorized according to different mechanisms [26,29]. The main advantage of mRNA vaccines is the simplicity of the vaccines as they only encode the gene of interest. mRNA can be easily mass-produced in vitro at a low production cost. The main limitation of the mRNA vaccine is stability. Many mRNA vaccines require cold or frozen storage. Various structural modification methods have been developed to increase the intracellular stability of the RNA molecules [30]. Recently developed mRNA and DNA vaccines against SARS-CoV-2 are the best examples of nucleic acid vaccines. Several mRNA vaccines are currently under development, with numerous mRNA vaccines are under Phases I–III clinical trials [28,30–32].
2.8. Viral vectored vaccines
Viral vectored vaccines are a newer technology similar to DNA vaccines, but use a harmless or attenuated virus to deliver the desired DNA into cells [33,34]. Genetic material from the microbe is loaded into carrier viruses. These loaded carrier viruses transport the microbial DNA into the cells. Recombinant vector vaccines mimic a natural infection and thus stimulate the immune system. Viral vectored vaccines are usually grown in cell lines and can easily be manufactured on a large scale. Compared to nucleic acid vaccines and recombinant subunit vaccines, viral vectored vaccines are significantly cheaper to manufacture. The best known example of a viral vectored vaccine is the SARS-CoV-2 vaccine, which was developed using adenovirus vector ChAdOx1 [35].
3. Modern rational vaccine engineering
Although significant advances have been made in vaccine development since the early intricate and poorly-defined preparations, the principles of vaccine design remain essentially the same. The most important element of a vaccine is the antigen. In order to induce robust antibody responses, it is essential to have a suitable antibody-inducing antigen. The ideal antigen candidate should contain antigenic epitopes for B-cells and epitope regions that can be recognized by T-cell receptors [36]. The structural features of the antigen can greatly impact the desired antibody-response. With advances in bioinformatics, systems biology, structural biology, and genomics, the features of vaccine components could be improved. For example, with structural vaccinology and reverse vaccinology approaches, promising antigenic epitope regions could be identified based on structural studies of the target epitopes of effective antibodies [37–39]. Using systems biology approaches, we can better understand host-pathogen interactions, immunological mechanisms of infection and human immune response to vaccines, which will lead to the development of improved vaccines [40].
Most of the traditional vaccines have some disadvantages: the use of native antigenic preparations in conventional vaccines has proven to be successful against a range of pathogens such as hepatitis B virus and poliovirus, in which surface antigens do not vary rapidly [41,42]. However, traditional vaccination methods have struggled to deliver successful vaccines against pathogens with high mutation rates. Vaccines with native antigen preparations are not always optimal [43]. Native surface proteins of pathogens are evolved to bypass the immunity, not to induce it [44]. The lack of highly expressed surface antigens is another reason that limits the application of traditional vaccine design. Traditional vaccines are certainly not suitable for stable storage, efficient production, or safe vaccination. Conventional vaccine approaches are not well suited for non-infectious diseases. Furthermore, viruses have a limited number of protective determinants, which necessitates the optimization of relatively a small set of antigenic proteins.
Major challenges in modern vaccine development are pathogens with high mutation rates. For examples, HIV, SARS-CoV-2, and hepatitis C viruses alter their surface antigens very rapidly and extensively. In addition to this, some of the requirements for modern vaccines are efficacy, rapid development strategies, and an effective platform for mass production. With the help of structural vaccinology and genomics approaches new antigenic regions on the pathogens that were previously not employed as targets of natural immunity could be identified and utilized in vaccine design. A rational, structure-driven approach can produce potent immunogens with atomic accuracy and epitope-specific targeting to induce prolonged protective immunity. Rationally produced vaccines are known to perform better in inducing a protective immune response. A rational, structure-guided vaccine design aids the design and development of structurally simpler immunogens that have well-defined minimal epitopes targeted by neutralizing antibodies. By optimally engineering the epitope, it is possible to enable broadly cross-protective epitopes. By combining the tools of genetic engineering and computational biology with structural vaccinology, optimized, effective, and stable immunogens can be developed. Such immunogens can be produced more efficiently and stably stored than the native molecules, which reduces the costs and removes the barrier distributions.
4. Structural vaccinology approach
In the B-cell-mediated response, the epitopes recognized by neutralizing antibodies are three-dimensional in nature [9]. The main mechanism of most vaccines is that these three-dimensional epitopes are presented to B-cells for clonal expansion and affinity maturation of the antibodies. On the other hand, in the T-cell-mediated immune response, the epitopes are mostly short peptides that lack a defined three-dimensional structure [45]. Therefore, knowing the three-dimensional structure of an antigen is not very important in the development of T-cell peptide-based vaccines. However, the knowledge of structural basis of antigen presentation by MHCs has provided much insight into antigenic peptide designs and this would enable more efficient antigen design to induce a T-cell-mediated response. Structural biology approaches have made it possible to determine the structure of antigenic proteins and antigen-antibody complexes. The critical structural information that these three-dimensional structures provide can be used in vaccine development. Immunogens are rationally designed using this structural information.
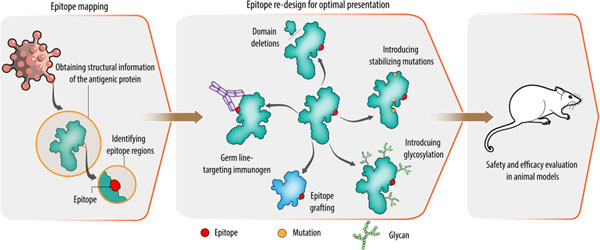
Structural vaccinology works on the principle that protective determinants on the pathogens can be used to selectively engineer the antigen in a stable and simpler form for inclusion in vaccine preparations [10]. This approach involves (i) determining atomic structure of the antigen and identifying epitope regions, (ii) redesigning the epitope by molecular engineering, (iii) safety and efficacy assessment of the designed vaccine candidates (Fig. 2). In the first step, the 3D structure of the antigen or the antigen–antibody complex is determined using experimental methods, such as X-ray crystallography, cryo-electron microscopy (cryo-EM), or nuclear magnetic resonance (NMR) methods. Obtaining the structural information provides details about the binding sites of antibodies on the antigens. It also provides useful information about the molecular nature of host-pathogen interactions and of pathogen- or vaccine-induced antibody responses. In addition, structural information of antigen-antibody complexes helps to define domain boundaries for epitopes and provides useful information for the engineering of the epitopes.
Figure 2. Structure-based approaches for immunogen design.
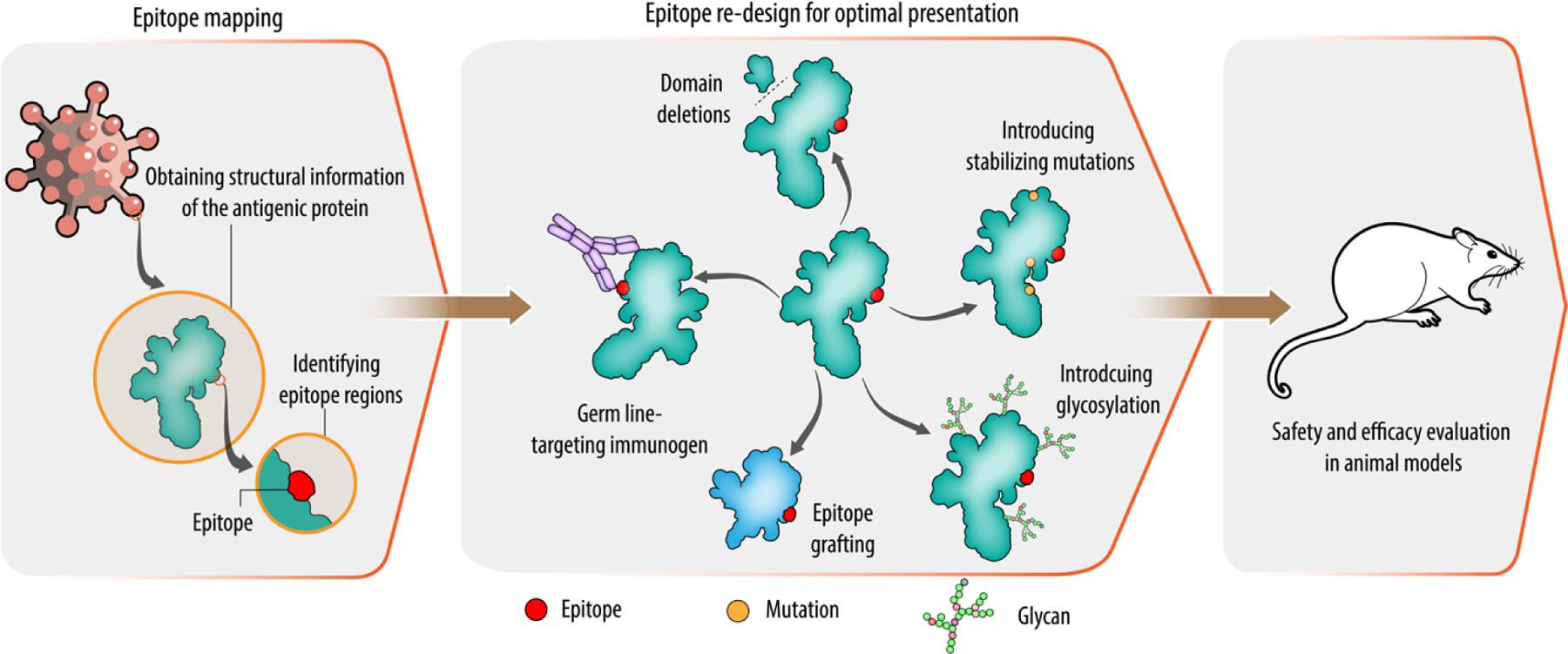
In order to identify potential epitopes, structural details of the antigen are obtained in the first step using computational and experimental methods. In the next step, identified epitopes are re-designed for an optimal presentation. Various approaches for epitope presentation are shown in the middle panel. In the last step, engineered epitopes are tested in animal models for efficacy and safety.
Among the three methods, X-ray crystallography is one of the most widely used techniques for epitope mapping [43,46]. Crystal structures enable a precise determination of the key interactions between individual amino acids from both side chains and main chain atoms in the antigen-antibody complex. Examples include high resolution structures of antibody complexes with hemagglutinin, HIV-1 Env protein and syncytial virus glycoprotein (Fig. 3) [47–49]. The immune epitope database (http://www.iedb.org/) stores a full list of available structures and epitopes [50]. Even with complex conformational epitopes, X-ray crystallography allows inspection of specific antibody-antigen interactions. However, X-ray crystallography is not the one-step solution for investigating all epitopes. Generation of crystals typically requires large amounts of very pure protein samples, and the entire process takes unpredictable time. At the same time, there will be uncertainty over getting diffraction quality crystals, which can often be the rate limiting step. With membrane proteins, the task can be even more difficult. Currently available software makes it possible to determine first models very quickly after collecting diffraction data.
Figure 3. Representative structures of antigen-antibody complex.
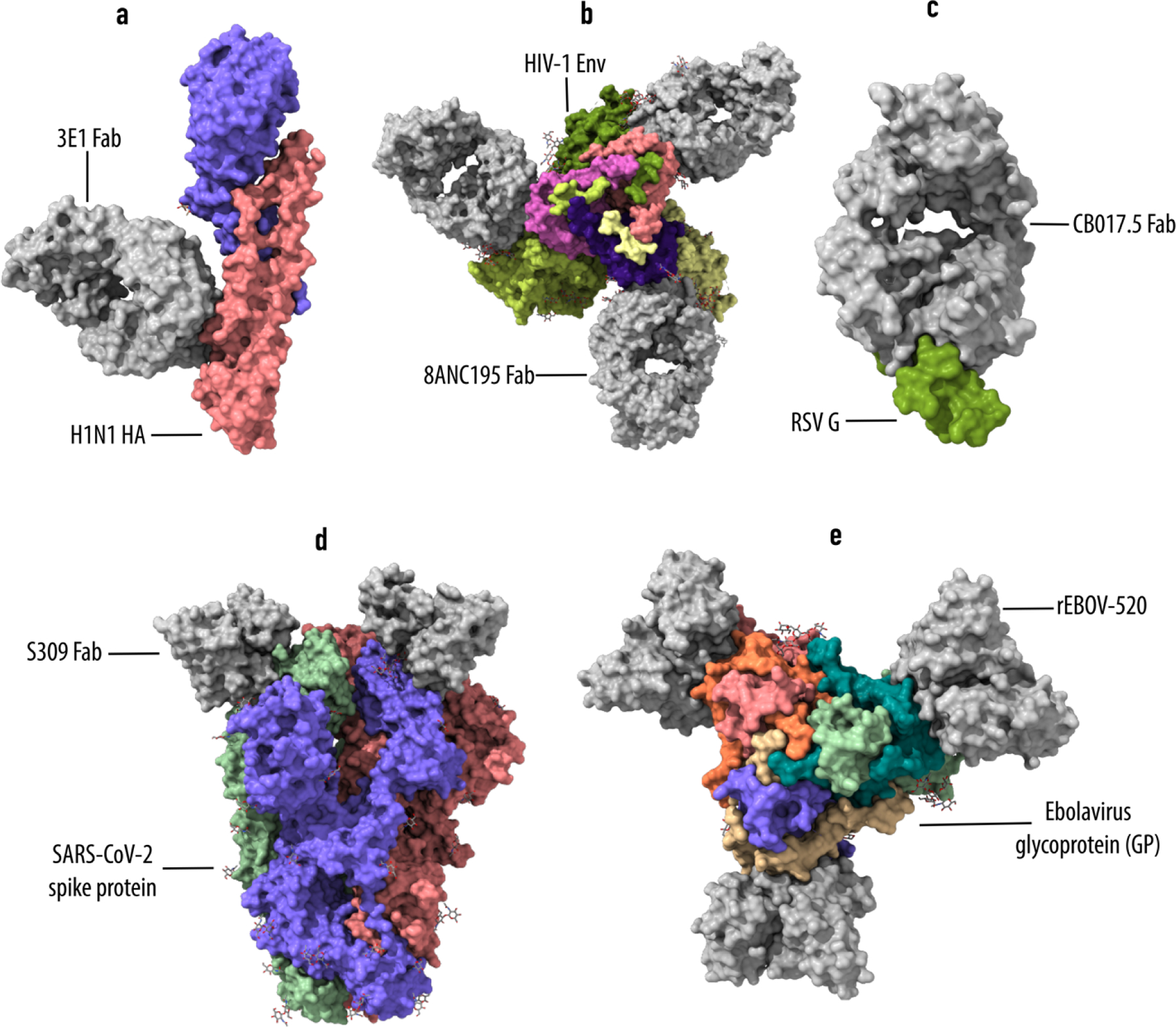
a, crystal structure of 3E1 Fab (fragment antigen binding) in complex with hemagglutinin (HA) of influenza A H1N1 (PDB: 5GJS). b, crystal structure of 8ANC195 Fab in complex with HIV-1 Env trimer (PDB: 5CJX). c, crystal structure of respiratory syncytial virus glycoprotein (RSV G) bound to Fab CB017.5 (PDB: 6BLH). d, Cryo-EM structure of the SARS-CoV-2 spike glycoprotein in complex with S309 Fab (PDB: 6WPT). e, Cryo-EM structure of Ebola virus glycoprotein in complex with rEBOV-520 Fab (PDB: 6PCI). Antibodies/Fabs are shown in grey.
Solution NMR could be one useful option to characterize antibody/antigen complexes. Solution NMR offers a rapid and accurate method for epitope mapping that is particularly useful for antigens which are <30 kDa [51,52]. Solution NMR spectroscopy is ideal for characterizing intermolecular interfaces such as antibody and antigen complexes. By mapping the NMR chemical shift, a detailed description of the binding footprint of one molecule over another could be obtained. NMR mapping can be useful for characterizing epitopes in a very short time. There are some limitations with the NMR epitope mapping. Larger antigens result in spectral overlap and signal broadening that resulting in loss of sensitivity. In addition, the target antigens must be labeled with NMR-active nuclei. Also, the time frame for NMR assignment process can vary and take up to several months. An example of NMR structure of the antigen and antibody complex is the variable fragment of 0.5b (HIV-1 neutralizing antibody), which is bound to the third hypervariable region (V3) of gp120 protein of HIV-1 [53].
Cryo-electron microscopy (Cryo-EM) is another method that has been used to determine macromolecular structures with a resolution that is sometimes comparable to X-ray crystallography [54]. Samples are flash frozen in cryo-EM; hence crystallization is not required. Typically, a much less samples are required but they still need to be relatively homogeneous in purity. Cryo-EM works best for the larger molecules to get higher resolution information and less information for smaller molecules. Examples of cryo-EM determined antigen-antibody complexes include structures of antibody complexes with SARS-CoV-2 spike glycoprotein and Ebola virus antigenic glycoprotein (Fig. 3) [55,56]. Apart from antigen-antibody complexes, cryo-EM has been successfully used to map the epitopes on several icosahedral viruses, which are generally difficult to crystallize due to their large size [57].
At present, X-ray crystallography is still the leading method for obtaining high-resolution information on the antigen-antibody interaction. However, single-particle cryo-EM has made it possible to resolve the three-dimensional structures of numerous proteins with atomic or near-atomic resolution.
Compared to experimental efforts, computational epitope prediction is efficient and inexpensive. In recent years, great efforts have been made to develop methods for predicting epitopes in silico and computational methods of mapping the epitopes have made significant advances. These studies are discussed in detail elsewhere [40]. The initial computational methods of epitope prediction were sequence-based and focused on predicting linear or continuous B-cell epitopes. With the availability of vastly expanded repertoire of structural data on antigen and antibody interactions, and a wealth of information on epitope-paratope interactions obtained through epitope mapping studies over the past few decades, machine learning and knowledge-based methods have been introduced to increase the accuracy and reliability of the predictions [58]. However, computational predictions could be challenging in the early stages of the investigation when structural information is limited or unavailable. Nonetheless, with the continued advances in computational tools for in silico prediction of 3D protein structures, more accurate 3D protein structures can be modeled. With the introduction of AlphaFold2, it is possible to predict protein structures with remarkable accuracy [59]. Despite all these efforts, the prediction of discontinuous epitopes is still challenging and requires an experimental 3D structure for validation.
5. Redesigning the epitope by molecular engineering
Once the structural details of the antigen protein are obtained, the antigen is redesigned for optimized epitope presentation. To develop an effective antigen, the immunological and bioinformatic knowledge must be combined synergistically with the structural knowledge. Typically the redesigning process involves stabilization of the immunogen conformation by introducing certain mutations, masking/removal of the unwanted epitope regions, optimization of the overall thermostability of the immunogen and introduction of additional epitopes (Fig. 4) [10,43]. Silencing non-neutralizing epitopes, conformational stabilization method, germline targeting approach, and epitope scaffolding are some of the approaches that are used to elicit a focused immune response [60].
Figure 4. Immunogen design using epitope-scaffold approach.
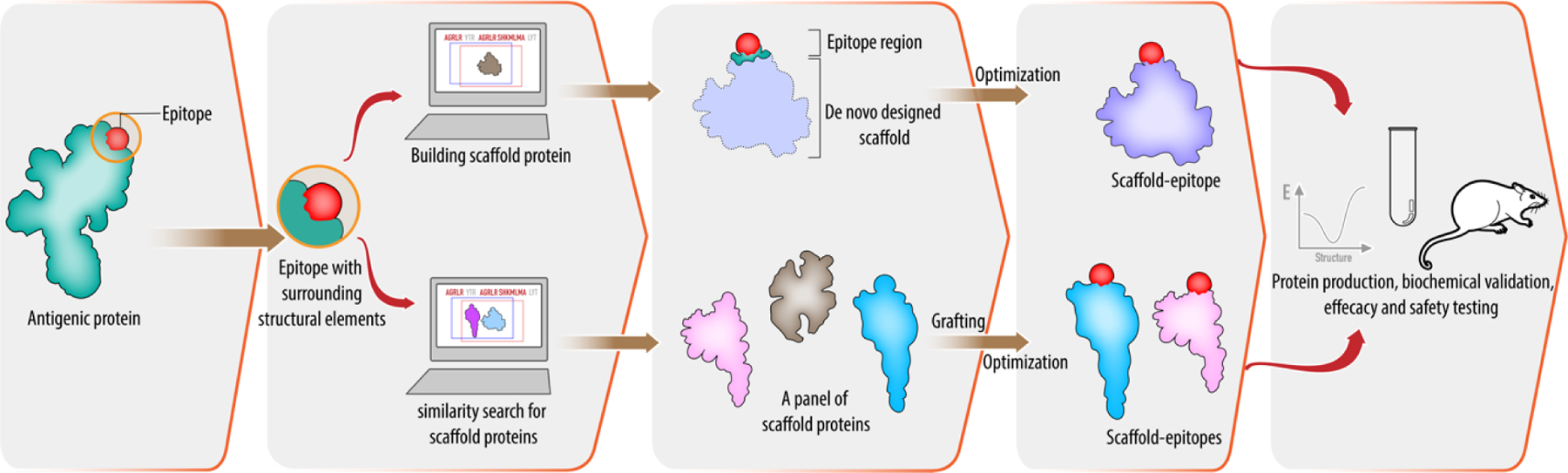
Structural components of epitopes are excised from the antigenic protein and the epitopes are either stabilized in de novo–designed scaffold proteins (panels 3 & 4, top) or a set of scaffold proteins is identified by PDB database scanning based on similarity search and the epitopes are stabilized by grafting (panels 3 & 4, bottom). Finally, engineered proteins are produced in a suitable system and tested in animal models.
In silencing non-neutralizing epitopes approach, non-neutralizing epitopes are either removed or masked using various methods. Removal of non-neutralizing domains is an aggressive approach of silencing non-neutralizing epitopes, where non-neutralizing epitope containing domains are completely excised from the protein to induce a focused immune response. For example, head-only fusion glycoprotein immunogens for RSV demonstrated increased antibody titers against neutralization epitopes of the head domain in an immunized mouse [61]. In contrast, masking is a less aggressive approach in which the non-functional epitope regions or domains are hidden from antigen receptors through hyperglycosylation of the masked region. For example, hyperglycosylation in influenza hemagglutinin restricted the antibody repertoire to an occluded, conserved epitope at the hemagglutinin head interface [62]. In germline targeting approach, immunogens are developed to activate the precursors of broadly neutralizing antibodies. In this way, activated precursor cells mature along a pathway to broadly neutralizing antibodies that can neutralize the pathogens. An example of germline targeting approach is that of Steichen et al., in which the HIV BG18 germline-targeting immunogen successfully expanded and diversified the BG18 precursors [63]. In the conformational stabilization approach, various methods are used to achieve antigenic conformational stabilization. Some of the methods include stabilization through improved hydrophobic core packing by introducing cavity filling mutations, improving overall stability of the protein by introducing stabilizing mutations and disulfides, and improving trimerization and expression yield by proline substitution [64–67]. In epitope-scaffold approach, structural information of the epitope conformation recognized by neutralizing antibodies is used to design heterologous proteins that mimic the conformation of the epitopes. For this review, we focus only on the epitope scaffolding approach.
The epitope-scaffold approach is a versatile and high-precision approach for vaccine development that shows a lot of promise [68,69]. In the epitope scaffolding approach, epitopes are transplanted onto completely unrelated scaffold proteins for conformational stabilization. More specifically, structural information of the precise target epitope conformation is used to design heterologous proteins that mimic structural features of the target epitope (Fig. 4). The heterologous protein mimetics will be structurally compatible with the antibody binding mode. These protein mimetics feature epitope surface of the antigen and lack other molecular features of the antigenic protein. A unique feature of this approach is the ability to elicit an immune response to cryptic immunogens, immunogens which show no immunogenicity when vaccinated with attenuated, inactivated, or killed pathogenic vaccine preparations [70]. However, one limitation in focusing on a single epitope is that the design could potentially fail due to the mutations in the epitope. Therefore, it is necessary to choose a highly conserved epitope across the strains rather than the variable region.
Initial studies on the application of epitope scaffold approach were reported by Correia et al., Azoitei et al., McLellan et al., and Ofek et al [69,71–74]. Correia et al. and Ofek et al. targeted the HIV epitopes 4E10 and 2F5 respectively [72,74]. The authors transplanted the epitopes onto small, non-viral scaffold proteins using the side-chain grafting method. In both cases, the transplanted proteins were stable and displayed high affinity binding against target antibodies. Crystal structures showed a high degree of epitope structural mimicry by the designed epitopes. In both studies, epitope-scaffolds were able to elicit a specific immunological response in tested animal models. One of the limitations with side-chain grafting is that it relies on the presence of scaffolds with structurally exposed backbone similar to the target epitope. To incorporate backbone flexibility modeling into grafting, Azoitei et al. developed a backbone grafting approach in which the backbone and side chains of linear functional motifs are transplanted onto scaffold proteins [73,75]. Authors tested this approach using the engineered HIV 2F5 epitope scaffolds, authors compared the backbone grafting methods with the side-chain grafting methods. Backbone grafted epitopes showed better affinity against the target antibody. McLellan et al. designed scaffold-epitopes displaying the antigenic site II of respiratory syncytial virus (RSV), which is a target of the monoclonal antibody palivizumab [71]. Even though the side-chain grafted protein mimetics showed high degree of epitope mimicry, these epitope-scaffolds failed to produce neutralizing antibodies when used as immunogens in mice.
Correia et al. developed a computational method called Fold From Loops (FFL) to allow de novo folding and design of scaffold proteins [69]. Using their computational method, authors designed scaffold-epitopes for the helix-turn-helix conformation of the mota epitope of RSV. From the biophysical, structural, and functional studies, authors showed that the small, thermally and conformably stable scaffold-epitopes accurately mimicked the mota epitopes and induced neutralizing activity in most of the immunized macaques. Zhu et al. designed epitope-scaffolds against HIV by computationally designing epitopes that mimic the surface features of carbohydrate-occluded neutralization epitopes (CONE) of Env protein through side chain grafting approach [76]. Zhu et al. used their computational methods for scaffold identification and grafting, and the resulting scaffold-epitopes reproduced the conformation of native target antigen [77–79]. Immunogenic evaluation in rabbits showed Env binding and neutralizing activity. To develop a broad immunity against influenza, Bajic et al. targeted the receptor-binding site (RBS), a conserved epitope on viral hemagglutinin (HA) [80]. Authors used non-circulating, avian influenza HAs as molecular scaffolds to display the RBS epitope from circulating H1 influenza. Upon immunization, RBS epitope-scaffolds elicited RBS-directed antibodies.
Recently, Hauser et al. used glycan engineering and scaffold based immunofocusing approach to graft the receptor binding motif (RBM) of the receptor binding domain (RBD) from spike protein of SARS-CoV-2 onto heterologous coronavirus RBDs [81]. When tested in murine model, authors observed improved targeting of the serum response and robust SARS-CoV-2 neutralizing activity across SARS-CoV-2 variants and other coronaviruses.
In summary, epitope-scaffolding approach is an efficient way to present the epitope surface to induce epitope-specific antibodies. However, there are certain challenges for the epitope-scaffolding approach. The main challenge is to graft and stabilize discontinuous epitopes as majority of antibody epitopes are discontinuous. These approaches mainly focus on B-cell responses. To generate better immune responses, the approach should be expanded to improve T-cell responses. Usually, a single epitope is included in structure-based vaccines. To improve the efficacy and the breadth of protection elicited by immunogen, multiple epitopes could be included (both B- and T-cell epitopes) in a single immunogen. This could be an ideal method to induce strong immunity or to protect against multiple strains of the pathogen. Such engineered immunogens could also be useful in diagnostic applications. A limiting factor in this approach is that it requires structural and immunological knowledge of antigen-induced immune response.
6. Concluding remarks
With the rapid advancements in protein engineering and antigen design, vaccinology has entered a new era. A combination of structural biology, reverse vaccinology and synthetic biology will play a major role in understanding the molecular mechanisms of the immune response of vaccines and transform vaccine development through increasingly rational design. Advanced computational approaches have been used for epitope prediction and its rational optimization for vaccine development. Computational tools will likely play an indispensable role in future structure-based vaccine design. As X-ray crystallography and cryo-EM advance, the number of antigen-antibody structures in the Protein Data Bank (PDB) is likely to increase, and this will provide a wealth of information for the development of computational tools for epitope mapping and immunogen design. Structure based vaccines combined with improved methods of antigen delivery such as liposomes, polymeric particles, inorganic particles and self-assembling nano particles have the potential to enhance the immune response to pathogens [29]. There are ongoing efforts to improve antigen delivery methods and rationally develop better adjuvants to improve immunogenicity. Recent developments in structure-based vaccine development approaches hold promise against emerging pathogens and against challenging targets where conventional approaches have been ineffective. As technology continues to improve, we expect novel vaccines in the coming years that target other diseases susceptible to immune modulation, such as cancer and neurodegenerative diseases. Currently, the average time for conventional vaccine development takes around 10 years, suggesting the need for fast development approaches for emerging outbreaks [82]. The COVID-19 pandemic has shown that vaccines can be developed at unprecedented speed with the use of advances in technology. In the days to come, we expect the advent of new and more effective vaccines as a result of implementing the emerging knowledge and technological advances.
Acknowledgements
We acknowledge support from the National Institutes for Health (1R35 GM134864) and the Passan Foundation.
Abbreviations
- APC
Antigen Presenting Cell
- RSV
respiratory syncytial virus
- VLP
Virus Like Particle
- HPV
Human Papillomavirus
- CONE
Carbohydrate-Occluded Neutralization Epitopes
- Cryo-EM
Cryo-Electron Microscopy
- NMR
Nuclear Magnetic Resonance
- RBS
Receptor-Binding Site
- RBM
Receptor-Binding Motif
- RBD
Receptor-Binding Domain
- HA
Hemagglutinin
- Fab
Fragment Antigen Binding
- MHC
Major Histocompatibility Complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
References
- [1].Pollard AJ, Bijker EM, A guide to vaccinology: from basic principles to new developments, Nat. Rev. Immunol 21 (2021) 83–100. 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moyer TJ, Zmolek AC, Irvine DJ, Beyond antigens and adjuvants: formulating future vaccines, J. Clin. Invest 126 (2016) 799–808. 10.1172/JCI81083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Gregorio E, Rappuoli R, From empiricism to rational design: A personal perspective of the evolution of vaccine development, Nat. Rev. Immunol 14 (2014) 505–514. 10.1038/nri3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mak TW, Saunders ME, B.D.B.T.-P. to the I.R. (Jett Second E., eds., Chapter 14 - Vaccination, in: Academic Cell, Boston, 2014: pp. 333–375. 10.1016/B978-0-12-385245-8.00014-5. [DOI] [Google Scholar]
- [5].Capecchi B, Serruto D, Adu-Bobie J, Rappuoli R, Pizza M, The genome revolution in vaccine research, Curr. Issues Mol. Biol 6 (2004). 10.21775/cimb.006.017. [DOI] [PubMed] [Google Scholar]
- [6].Afrough B, Dowall S, Hewson R, Emerging viruses and current strategies for vaccine intervention, Clin. Exp. Immunol 196 (2019) 157–166. 10.1111/cei.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tannock GA, Kim H, Xue L, Why are vaccines against many human viral diseases still unavailable; an historic perspective?, J. Med. Virol 92 (2020) 129–138. 10.1002/jmv.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rinaudo CD, Telford JL, Rappuoli R, Seib KL, Vaccinology in the genome era, J. Clin. Invest 119 (2009) 2515–2525. 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dormitzer PR, Ulmer JB, Rappuoli R, Structure-based antigen design: a strategy for next generation vaccines, Trends Biotechnol 26 (2008) 659–667. 10.1016/j.tibtech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anasir MI, Poh CL, structural vaccinology for viral vaccine design, Front. Microbiol 10 (2019) 738. 10.3389/fmicb.2019.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Minor PD, Live attenuated vaccines: Historical successes and current challenges, Virology 479–480 (2015) 379–392. 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- [12].Bhardwaj S, Chapter 21 - Vaccines, in: Vohora D, G.B.T.-P.M. and Singh TCR (Eds.), Academic Press, Boston, 2018: pp. 341–353. 10.1016/B978-0-12-802103-3.00022-5. [DOI] [Google Scholar]
- [13].Yadav DK, Yadav N, Khurana SMP, Vaccines: Present status and applications, Elsevier, 2013. 10.1016/B978-0-12-416002-6.00026-2. [DOI] [Google Scholar]
- [14].Karch CP, Burkhard P, Vaccine technologies: From whole organisms to rationally designed protein assemblies, Biochem. Pharmacol 120 (2016) 1–14. 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clem AS, Fundamentals of vaccine immunology, J. Glob. Infect. Dis 3 (2011) 73–78. 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sanders B, Koldijk M, Schuitemaker H, Inactivated viral vaccines, Vaccine Anal. Strateg. Princ. Control (2014) 45–80. 10.1007/978-3-662-45024-6_2. [DOI] [Google Scholar]
- [17].D’Amico C, Fontana F, Cheng R, Santos HA, Development of vaccine formulations: past, present, and future, Drug Deliv. Transl. Res 11 (2021) 353–372. 10.1007/s13346-021-00924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M, Understanding modern-day vaccines: what you need to know, Ann. Med 50 (2018) 110–120. 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- [19].Thomas S, Luxon BA, Vaccines based on structure-based design provide protection against infectious diseases, Expert Rev. Vaccines 12 (2013) 1301–1311. 10.1586/14760584.2013.840092. [DOI] [PubMed] [Google Scholar]
- [20].Pichichero ME, Protein carriers of conjugate vaccines: characteristics, development, and clinical trials, Hum. Vaccin. Immunother 9 (2013) 2505–2523. 10.4161/hv.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, Ahmadian G, Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers, J. Nanobiotechnology 19 (2021) 1–27. 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Noad R, Roy P, Virus-like particles as immunogens, Trends Microbiol 11 (2003) 438–444. 10.1016/S0966-842X(03)00208-7. [DOI] [PubMed] [Google Scholar]
- [23].Zhang LF, Zhou J, Chen S, Cai LL, Bao QY, Zheng FY, Lu JQ, Padmanabha J, Hengst K, Malcolm K, Frazer IH, HPV6b virus like particles are potent immunogens without adjuvant in man, Vaccine 18 (2000) 1051–1058. 10.1016/S0264-410X(99)00351-5. [DOI] [PubMed] [Google Scholar]
- [24].Vogel FR, Sarver N, Nucleic acid vaccines, Clin. Microbiol. Rev 8 (1995) 406–410. 10.1128/CMR.8.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu MA, Wahren B, Hedestam GBK, DNA Vaccines: Recent developments and future possibilities, Hum. Gene Ther 17 (2006) 1051–1061. 10.1089/hum.2006.17.1051. [DOI] [PubMed] [Google Scholar]
- [26].Qin F, Xia F, Chen H, Cui B, Feng Y, Zhang P, Chen J, Luo M, A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: from basic principles to current applications, Front. Cell Dev. Biol 9 (2021) 1–13. 10.3389/fcell.2021.633776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Suschak JJ, Williams JA, Schmaljohn CS, Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity, Hum. Vaccines Immunother 13 (2017) 2837–2848. 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pardi N, Hogan MJ, Porter FW, Weissman D, mRNA vaccines-a new era in vaccinology, Nat. Rev. Drug Discov 17 (2018) 261–279. 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wallis J, Shenton DP, Carlisle RC, Novel approaches for the design, delivery and administration of vaccine technologies, Clin. Exp. Immunol 196 (2019) 189–204. 10.1111/cei.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sahin U, Karikó K, Türeci Ö, mRNA-based therapeutics — developing a new class of drugs, Nat. Rev. Drug Discov 13 (2014) 759–780. 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- [31].Kauffman KJ, Webber MJ, Anderson DG, Materials for non-viral intracellular delivery of messenger RNA therapeutics, J. Control. Release 240 (2016) 227–234. 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- [32].Grunwitz C, Kranz LM, mRNA Cancer Vaccines—Messages that Prevail BT - Cancer Vaccines, in: Savelyeva N, Ottensmeier C (Eds.), Springer International Publishing, Cham, 2017: pp. 145–164. 10.1007/82_2017_509. [DOI] [PubMed] [Google Scholar]
- [33].Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AVS, Viral vectors as vaccine platforms: deployment in sight, Curr. Opin. Immunol 23 (2011) 377–382. 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [34].Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AVS, Dorrell L, Viral vectors as vaccine platforms: from immunogenicity to impact, Curr. Opin. Immunol 41 (2016) 47–54. 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- [35].Lundstrom K, Viral vectors for covid-19 vaccine development, Viruses 13 (2021). 10.3390/v13020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Burton DR, Antibodies, viruses and vaccines, Nat. Rev. Immunol 2 (2002) 706–713. 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- [37].Rappuoli R, Bottomley MJ, D’Oro U, Finco O, De Gregorio E, Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design, J. Exp. Med 213 (2016) 469–481. 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burton DR, What are the most powerful immunogen design vaccine strategies? reverse vaccinology 2.0 shows great promise, Cold Spring Harb. Perspect. Biol 9 (2017) a030262. 10.1101/cshperspect.a030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Graham BS, Advances in antiviral vaccine development, Immunol. Rev 255 (2013) 230–242. 10.1111/imr.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sunita A. Sajid, Singh Y, Shukla P, Computational tools for modern vaccine development, Hum. Vaccines Immunother 16 (2020) 723–735. 10.1080/21645515.2019.1670035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pattyn J, Hendrickx G, Vorsters A, Van Damme P, Vaccines Hepatitis B, J. Infect. Dis 224 (2021) S343–S351. 10.1093/infdis/jiaa668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baicus A, History of polio vaccination, World J. Virol 1 (2012) 108–114. 10.5501/wjv.v1.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liljeroos L, Malito E, Ferlenghi I, Bottomley MJ, Structural and computational biology in the design of immunogenic vaccine antigens, J. Immunol. Res 2015 (2015). 10.1155/2015/156241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mak TW, Saunders ME, Immunity to Pathogens, The Immune Response (2006) 641–694. 10.1016/B978-012088451-3.50024-7. [DOI] [Google Scholar]
- [45].Purcell AW, McCluskey J, Rossjohn J, More than one reason to rethink the use of peptides in vaccine design, Nat. Rev. Drug Discov 6 (2007) 404–414. 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- [46].Toride King M, Brooks CL, Epitope mapping of antibody-antigen interactions with x-ray crystallography, Methods Mol. Biol 1785 (2018) 13–27. 10.1007/978-1-4939-7841-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang W, Sun X, Li Y, Su J, Ling Z, Zhang T, Wang F, Zhang H, Chen H, Ding J, Sun B, Human antibody 3E1 targets the HA stem region of H1N1 and H5N6 influenza A viruses, Nat. Commun 7 (2016) 13577. 10.1038/ncomms13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scharf L, Wang H, Gao H, Chen S, McDowall AW, Bjorkman PJ, Broadly Neutralizing Antibody 8ANC195 Recognizes Closed and Open States of HIV-1 Env, Cell 162 (2015) 1379–1390. 10.1016/j.cell.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jones HG, Ritschel T, Pascual G, Brakenhoff JPJ, Keogh E, Furmanova-Hollenstein P, Lanckacker E, Wadia JS, Gilman MSA, Williamson RA, Roymans D, van ‘t Wout AB, Langedijk JP, McLellan JS, Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies, PLoS Pathog 14 (2018) e1006935–e1006935. 10.1371/journal.ppat.1006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, Wheeler DK, Sette A, Peters B, The Immune Epitope Database (IEDB): 2018 update, Nucleic Acids Res 47 (2019) D339–D343. 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gershoni JM, Roitburd-Berman A, Siman-Tov DD, Tarnovitski Freund N, Weiss Y, Epitope mapping: the first step in developing epitope-based vaccines, BioDrugs 21 (2007) 145–156. 10.2165/00063030-200721030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bardelli M, Livoti E, Simonelli L, Pedotti M, Moraes A, Valente AP, Varani L, Epitope mapping by solution NMR spectroscopy, J. Mol. Recognit 28 (2015) 393–400. 10.1002/jmr.2454. [DOI] [PubMed] [Google Scholar]
- [53].Tugarinov V, Zvi A, Levy R, Hayek Y, Matsushita S, Anglister J, NMR structure of an anti-gp120 antibody complex with a V3 peptide reveals a surface important for co-receptor binding, Structure 8 (2000) 385–395. 10.1016/S0969-2126(00)00119-2. [DOI] [PubMed] [Google Scholar]
- [54].Malito E, Carfi A, Bottomley MJ, Protein crystallography in vaccine research and development, Int. J. Mol. Sci 16 (2015) 13106–13140. 10.3390/ijms160613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, Peter A, Guarino B, Spreafico R, Cameroni E, Case JB, Chen RE, Havenar-Daughton C, Snell G, Telenti A, Virgin HW, Lanzavecchia A, Diamond MS, Fink K, Veesler D, Corti D, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature 583 (2020) 290–295. 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- [56].Gilchuk P, Murin CD, Milligan JC, Cross RW, Mire CE, Ilinykh PA, Huang K, Kuzmina N, Altman PX, Hui S, Gunn BM, Bryan AL, Davidson E, Doranz BJ, Turner HL, Alkutkar T, Flinko R, Orlandi C, Carnahan R, Nargi R, Bombardi RG, Vodzak ME, Li S, Okoli A, Ibeawuchi M, Ohiaeri B, Lewis GK, Alter G, Bukreyev A, Saphire EO, Geisbert TW, Ward AB, Crowe JE Jr, Analysis of a therapeutic antibody cocktail reveals determinants for cooperative and broad ebolavirus neutralization, Immunity 52 (2020) 388–403.e12. 10.1016/j.immuni.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li N, Li Z, Fu Y, Cao S, Cryo-EM Studies of Virus-antibody immune complexes, Virol. Sin 35 (2020) 1–13. 10.1007/s12250-019-00190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang W, Niu Y, Xiong Y, Ke M, Prediction of conformational B-Cell Epitopes, Immunoinformatics, in: De RK, Tomar N (Eds.), Springer New York, New York, NY, 2014: pp. 185–196. 10.1007/978-1-4939-1115-8_10. [DOI] [PubMed] [Google Scholar]
- [59].Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D, Highly accurate protein structure prediction with AlphaFold, Nature 596 (2021) 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ward AB, Wilson IA, Innovations in structure-based antigen design and immune monitoring for next generation vaccines, Curr. Opin. Immunol 65 (2020) 50–56. 10.1016/j.coi.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Boyington JC, Joyce MG, Sastry M, Stewart-Jones GBE, Chen M, Kong W-P, Ngwuta JO, V Thomas P, Tsybovsky Y, Yang Y, Zhang B, Chen L, Druz A, Georgiev IS, Ko K, Zhou T, Mascola JR, Graham BS, Kwong PD, Structure-Based Design of Head-Only Fusion Glycoprotein Immunogens for Respiratory Syncytial Virus, PLoS One 11 (2016) e0159709. 10.1371/journal.pone.0159709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bajic G, Maron MJ, Adachi Y, Onodera T, McCarthy KR, McGee CE, Sempowski GD, Takahashi Y, Kelsoe G, Kuraoka M, Schmidt AG, Influenza antigen engineering focuses immune responses to a subdominant but broadly protective viral epitope, Cell Host Microbe 25 (2019) 827–835.e6. 10.1016/j.chom.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Steichen JM, Lin Y-C, Havenar-Daughton C, Pecetta S, Ozorowski G, Willis JR, Toy L, Sok D, Liguori A, Kratochvil S, Torres JL, Kalyuzhniy O, Melzi E, Kulp DW, Raemisch S, Hu X, Bernard SM, Georgeson E, Phelps N, Adachi Y, Kubitz M, Landais E, Umotoy J, Robinson A, Briney B, Wilson IA, Burton DR, Ward AB, Crotty S, Batista FD, Schief WR, A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses, Science 366 (2019) eaax4380. 10.1126/science.aax4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].S.R. W., Mika V, Norbert S, Aditi M, Linnea S, Roopa K, Maciej P, Ben B, M.P. J., O.W. C., Min L, M.J. P., Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1, J. Virol 76 (2002) 8875–8889. 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Do Kwon Y, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang G-Y, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O’Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GBE, Stuckey J, V Thomas P, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schön A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, McDermott AB, Mascola JR, Kwong PD, Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env, Nat. Struct. Mol. Biol 22 (2015) 522–531. 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].M.J. S., Man C, Gordon JM, Mallika S, S.-J.G.B. E., Yongping Y, Baoshan Z, Lei C, Sanjay S, Anqi Z, Tongqing Z, G.K. W., Azad K, Syed M, B.J. C., Gwo-Yu C, Cinque S, Ulrich B, B.A. Q., Hergen S, Tim B, Zizheng Z, Ningshao X, Sung-Youl K, John-Paul T, Srinivas R, G.B. S., K.P. D., Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus, Science (80-. ) 342 (2013) 592–598. 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, Langedijk JPM, A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism, Nat. Commun 6 (2015) 8143. 10.1038/ncomms9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kulp DW, Schief WR, Advances in structure-based vaccine design, Curr. Opin. Virol 3 (2013) 322–331. 10.1016/j.coviro.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, Connell MJ, Stevens E, Schroeter A, Chen M, MacPherson S, Serra AM, Adachi Y, Holmes MA, Li Y, Klevit RE, Graham BS, Wyatt RT, Baker D, Strong RK, Crowe JE, Johnson PR, Schief WR, Proof of principle for epitope-focused vaccine design, Nature 507 (2014) 201–206. 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Oscherwitz J, The promise and challenge of epitope-focused vaccines, Hum. Vaccines Immunother 12 (2016) 2113–2116. 10.1080/21645515.2016.1160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McLellan JS, Correia BE, Chen M, Yang Y, Graham BS, Schief WR, Kwong PD, Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus, J. Mol. Biol 409 (2011) 853–866. 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD, Elicitation of structure-specific antibodies by epitope scaffolds, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 17880–17887. 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Azoitei ML, Ban YEA, Julien JP, Bryson S, Schroeter A, Kalyuzhniy O, Porter JR, Adachi Y, Baker D, Pai EF, Schief WR, Computational design of high-affinity epitope scaffolds by backbone grafting of a linear epitope, J. Mol. Biol 415 (2012) 175–192. 10.1016/j.jmb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Correia BE, Ban YEA, Holmes MA, Xu H, Ellingson K, Kraft Z, Carrico C, Boni E, Sather DN, Zenobia C, Burke KY, Bradley-Hewitt T, Bruhn-Johannsen JF, Kalyuzhniy O, Baker D, Strong RK, Stamatatos L, Schief WR, Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope, Structure 18 (2010) 1116–1126. 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [75].Azoitei ML, C.B. E., Andrew BY-E, Chris C, Oleksandr K, Lei C, Alexandria S, Po-Ssu H, M.J. S., K.P. D., David B, S.R. K., S.W. R., Computation-Guided Backbone Grafting of a Discontinuous Motif onto a Protein Scaffold, Science (80-. ) 334 (2011) 373–376. 10.1126/science.1209368. [DOI] [PubMed] [Google Scholar]
- [76].Zhu C, Dukhovlinova E, Council O, Ping L, Faison EM, Prabhu SS, Potter EL, Upton SL, Yin G, Fay JM, Kincer LP, Spielvogel E, Campbell SL, Benhabbour SR, Ke H, Swanstrom R, Dokholyan NV, Rationally designed carbohydrate-occluded epitopes elicit HIV-1 Env-specific antibodies, Nat. Commun 10 (2019) 1–10. 10.1038/s41467-019-08876-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shirvanyants D, Alexandrova AN, Dokholyan NV, Rigid substructure search, Bioinformatics 27 (2011) 1327–1329. 10.1093/bioinformatics/btr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ding F, Tsao D, Nie H, Dokholyan NV, Ab initio folding of proteins with all-atom discrete molecular dynamics, Structure 16 (2008) 1010–1018. 10.1016/j.str.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yin S, Ding F, Dokholyan NV, Eris: an automated estimator of protein stability., Nat. Methods 4 (2007) 466–467. 10.1038/nmeth0607-466. [DOI] [PubMed] [Google Scholar]
- [80].Bajic G, Maron MJ, Caradonna TM, Tian M, Mermelstein A, Fera D, Kelsoe G, Kuraoka M, Schmidt AG, Structure-guided molecular grafting of a complex broadly neutralizing viral epitope, ACS Infect. Dis 6 (2020) 1182–1191. 10.1021/acsinfecdis.0c00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hauser BM, Sangesland M, Denis KJ St., Windsor IW, Feldman J, Lam EC, Kannegieter T, Balazs AB, Lingwood D, Schmidt AG, Rationally designed immunogens enable immune focusing to the SARS-CoV-2 receptor binding motif, BioRxiv (2021) 2021.03.15.435440. [Google Scholar]
- [82].Rauch S, Jasny E, Schmidt KE, Petsch B, New vaccine technologies to combat outbreak situations, Front. Immunol 9 (2018). 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]


