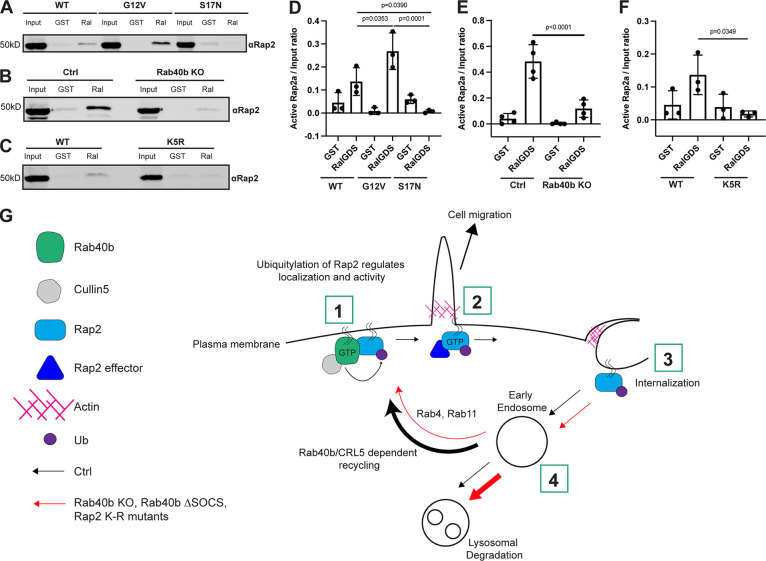
Figure 9.
Activation of Rap2 is regulated by Rab40b and is linked to its ubiquitylation. (A) Activation assay Rap2a-WT, -G12V, and -S17N. MDA-MB-231 parental cells were transiently transfected with GFP-Rap2a-WT, -G12V, or -S17N (pLVX-GFP constructs). Lysates were incubated with either GST or GST-RBDRalGDS, followed by a GST pull-down assay. GST alone was used to control for GST binding to Rap2a. 25 µg of lysate input was loaded as a positive control and used to calculate active levels of Rap2a. (B) Activation assay Rap2a-WT in Ctrl versus Rab40b KO cells. MDA-MB-231 parental cells or Rab40b KO cells were transiently transfected with GFP-Rap2a-WT (pEGFP-construct). Lysates were incubated with either GST or GST-RBDRalGDS, followed by a GST pull-down assay. GST alone was used to control for GST binding to Rap2a. 25 µg of lysate input was loaded as a positive control and used to calculate active levels of Rap2a. (C) Activation assay Rap2a-WT and -K5R. MDA-MB-231 parental cells were transiently transfected with GFP-Rap2a-WT and GFP-Rap2a-K5R (pLVX-GFP constructs). Lysates were incubated with either GST or GST-RBDRalGDS, followed by a GST pull-down assay. GST alone was used to control for GST binding to Rap2a. 25 µg of lysate input was loaded as a positive control and used to calculate active levels of Rap2a. (D) Quantification of activation assay in A. Three biological replicates were performed. Mean ± SD. Active Rap2a/input ratio was defined as active Rap2 signal/lysate signal. One-way ANOVA with Tukey’s multiple comparisons test. GST signal was not subtracted. WT versus G12V, P = 0.0353; WT versus S17N, P = 0.0390; G12V versus S17N, P = 0.0001. (E) Quantification of activation assay in B. Three biological replicates were performed. Mean ± SD. Active Rap2a/Input ratio was defined as active Rap2 signal/lysate signal. One-way ANOVA with Tukey’s multiple comparisons test. GST signal was not subtracted. Ctrl versus Rab40b KO, P < 0.0001. (F) Quantification of activation assay in C. Three biological replicates were performed. Mean ± SD. Active Rap2a/input ratio was defined as active Rap2 signal/lysate signal. One-way ANOVA with Tukey’s multiple comparisons test. GST signal was not subtracted. WT versus K5R, P = 0.0349. (G) Working model for how Rab40b/Cul5 modulates Rap2 subcellular localization and activation via ubiquitylation. (1) Rab40b/Cul5 and Rap2 primarily interact at the plasma membrane, where binding is enhanced when Rab40b is GTP bound. Ubiquitylation by the Rab40b/Cul5 complex causes dissociation. (2) Active Rap2 is then able to interact with its effector proteins at the plasma membrane, where it regulates leading-edge actin dynamics and ultimately drives cell migration. (3) To quickly re-distribute Rap2 to other locations at the lamellipodia plasma membrane, Rap2 is internalized via a pinocytosis-like mechanism. Internalization mediates the delivery of Rap2 to early endosomes. (4) Once at early endosomes, previous ubiquitylation by the Rab40b/Cul5 complex serves as a signal for Rap2 to be recycled back to the leading-edge plasma membrane via the Rab4/Rab11 recycling pathways. Without this ubiquitin signal, Rap2 is sorted to lysosomes for degradation. We hypothesize that Rap2 is continuously recycled to and from the plasma membrane to regulate its localization and activity. Ultimately, we propose that dynamic redistribution of Rap2 is required to maintain its enrichment at the leading edge, where it can regulate actin dynamics and promote cell migration. Source data are available for this figure: SourceData F9.