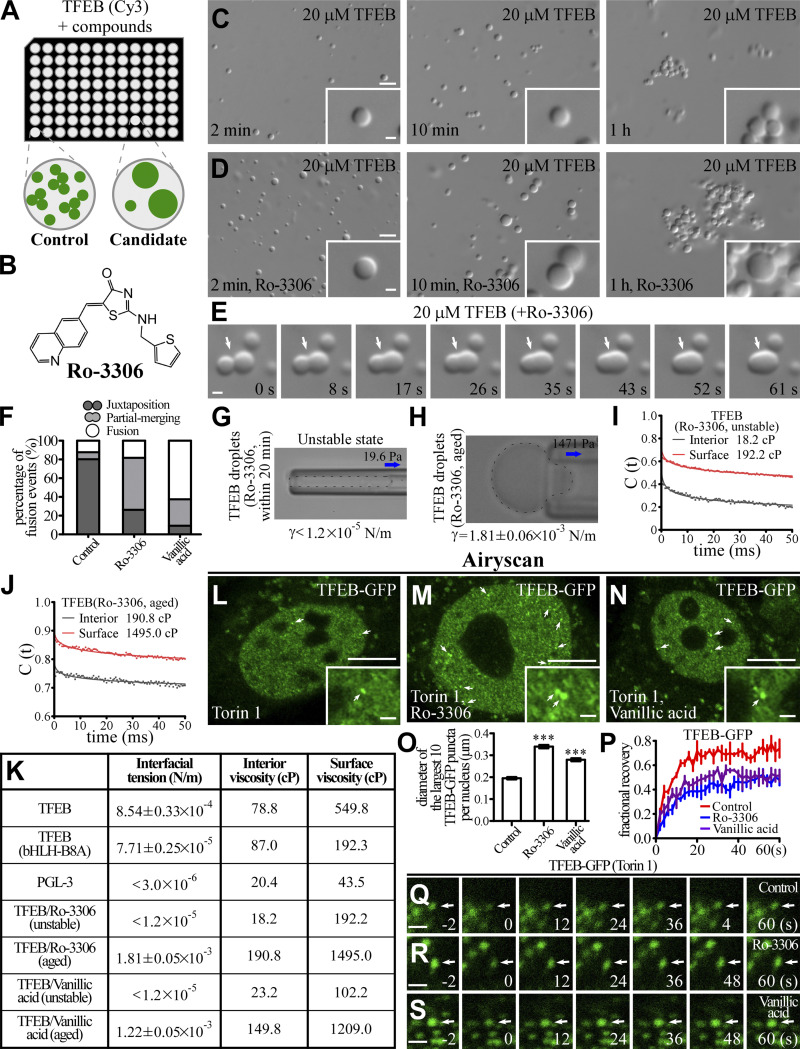
Figure 4.
Ro-3306 changes the material properties of TFEB condensates. (A) Schematic illustration of the strategy for screening small molecular compounds that increase the size of TFEB droplets. (B) 2D structure of Ro-3306. (C and D) DIC images showing that 20 μM TFEB forms droplets in 500 mM NaCl buffer with or without 50 μM Ro-3306 2 min to 1 h after LLPS induction. (E) Time-lapse images of two encountering TFEB droplets undergoing fusion (arrows) in buffer containing 50 μM Ro-3306. The time point of the first image is defined as 0 s. (F) Percentage of fusion events for droplets formed by 20 μM TFEB in the presence of 50 μM Ro-3306 and vanillic acid in 500 mM NaCl buffer. Three types of events (juxtaposition, partial merging, and fusion) are defined for droplets that underwent an encounter for 30 s. The control data are for TFEB droplets formed in 500 mM NaCl, as shown in Fig. 1 L. 126 and 120 fusion events were analyzed for TFEB/Ro-3306 and TFEB/vanillic acid droplets, respectively. (G and H) Representative pictures of the micropipette experiments for newly formed (G) and aged (H) TFEB droplets formed in 500 mM NaCl buffer containing 100 μM Ro-3306. 19.6 and 1,471 Pa of negative pressure was used to draw out the surface of newly formed and aged TFEB droplets, respectively. The direction of suction is indicated by arrows. The interfacial tension for newly formed droplets is estimated and shown underneath, and the calculated interfacial tension for aged droplets is shown underneath as mean ± SEM (n = 10). The droplet in G was not still and moved into the micropipette with time. (I and J) Autocorrelation decay curves (solid lines) reflect the rotational behavior of AuNRs in the droplets. The calculated apparent viscosity, which is corrected with the viscosity of the solution, is shown at the top. Rotational tracks of the AuNRs in the interior and on the surface were obtained from the same newly formed (I) and aged (J) TFEB droplets formed in 500 mM NaCl buffer containing 100 μM Ro-3306. (K) Summary of the measured interfacial tension and interior and surface viscosity of in vitro–assembled TFEB, TFEB(bHLH-B8A), PGL-3, and unstable and aged TFEB/Ro-3306 and TFEB/vanillic acid droplets. (L–O) Compared with control HeLa cells stably expressing TFEB-GFP (L), nuclear TFEB-GFP puncta are evidently enlarged by incubation in 10 μM Ro-3306 (M) and vanillic acid (N). Representative TFEB-GFP puncta are pointed out by arrows in L and M. Airyscan images are shown. To facilitate the analysis, cells were treated with Torin 1 to increase the nuclear TFEB level. The few punctate structures formed by TFEB-GFP in the cytoplasm upon Torin 1 treatment correspond to TFEB that is retained on lysosomes (L–N; Martina and Puertollano, 2013). Quantification of the diameter of the largest 10 TFEB-GFP puncta per nucleus in L–N (O). Data are shown as mean ± SEM (n = 100 puncta for each bar) in O. ***, P < 0.001. (P–S) FRAP analysis of the TFEB-GFP–positive punctate structures (arrows) in the nucleus of control HeLa cells (Q) and HeLa cells treated with 10 μM Ro-3306 (R) and vanillic acid (S). All cells were cotreated with Torin 1. Quantification of FRAP data for Q–S (P). Data are shown as mean ± SEM (n = 3) in P. Scale bars: 10 μm (L–N); 5 μm (C and D); 1 μm (E, Q–S, and insets in C, D, and L–N).