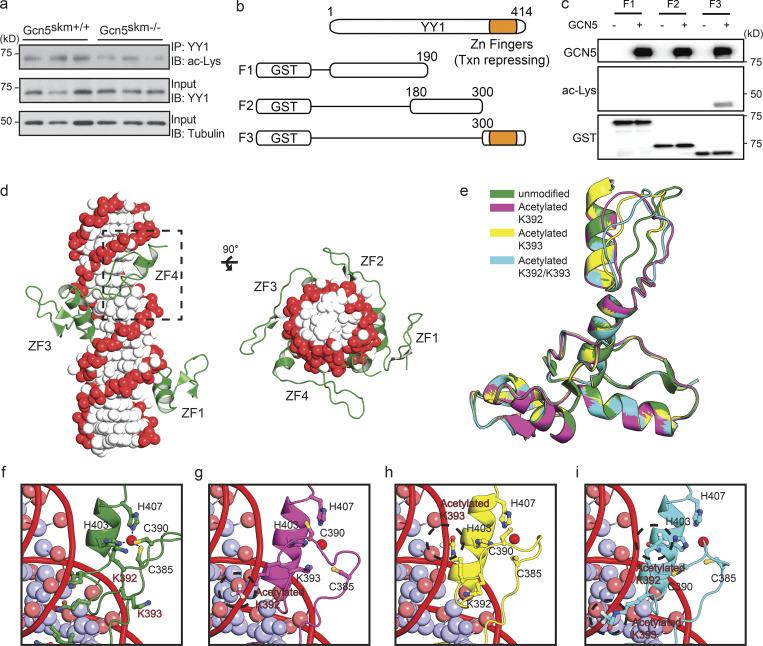
Figure 6.
GCN5 acetylates YY1 and alters a predicted model of YY1 binding to DNA. (a) Western blot (IB) of anti-YY1 immunoprecipitated (IP) from controls and Gcn5skm−/− mice probed with anti-acetyl lysine (ac-Lys; top). Control blots for IP YY1 probed with anti-YY1 (middle) and total protein input for IP probed with anti-tubulin (bottom). (b) Schematic showing GST-tagged truncated YY1 used for in vitro acetylation (Fig. 6 c) and MS analysis. (c) In vitro acetylation assay of the in vitro synthesized GST-YY1 fragments incubated with GCN5. YY1 fragments were incubated alone or with GCN5 (top). Acetylation of YY1-fragment (aa 300–414) detected with anti-acetyl lysine (ac-Lys; middle). Input YY1 fragments detected by anti-GST antibody (bottom). (d) Modeled structure of the YY1 zinc finger region bound to DNA. The DNA phosphate backbone and bases are colored red and white, respectively. The human YY1 protein is represented in green. The fourth zinc finger domain is boxed with a dotted line. (e–i) Modeling of the unmodified or acetylated (K392, K393, or K392/393) forms of the fourth zinc finger domain of YY1 and interactions with DNA. The residues involved in coordinating zinc ion, C385, C390, H403, and H407 are shown. The zinc ions are represented by red spheres. The acetylated forms of K392 and K393 are circled with a dotted line.