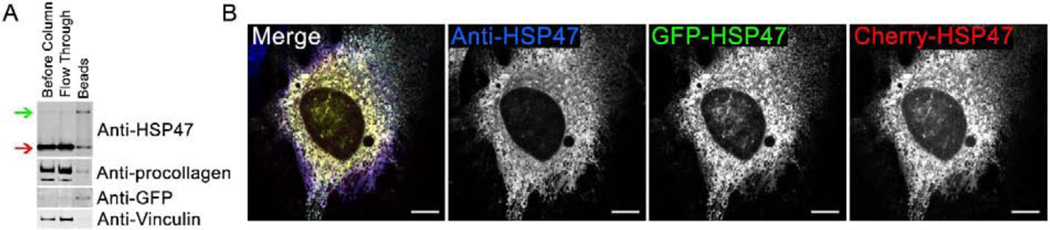
Fig. 1. Fluorescent protein-tagged HSP47 (FP-HSP47) interacts with procollagen and colocalizes with endogenous HSP47.
(A) Western blots of cell lysates before and after IP of GFP-HSP47 with Anti-GFP beads. Green arrow shows transfected GFP-HSP47; red arrow shows endogenous HSP47. Significant enrichment of procollagen relative to vinculin on the beads revealed that it co-immunoprecipitated with GFP-HSP47. Since the estimated procollagen concentration in cell lysates was lower than 1 μM and HSP47 binds procollagen with Kd ~ 1–10 μM [30], the co-immunoprecipitation indicated minimal or no disruption of HSP47-procollagen interaction by the fluorescent tag in GFP-HSP47. Because multiple endogenous HSP47 and GFP-HSP47 molecules would be bound to the same procollagen triple helix, a significant amount of endogenous HSP47 was found on the Anti-GFP beads. (B) Confocal imaging of colocalization between two FP-HSP47 constructs co-transfected into MC3T3 cells and HSP47 antibodies that label both transfected and endogenous HSP47. All spots labeled with anti-HSP47 were also positive for GFP-HSP47 and Cherry-HSP47, indicating complete colocalization of transfected and endogenous molecules in all subcellular compartments. Variations in relative fluorescence intensity of different tags are affected by antibody accessibility, uneven photobleaching during sample preparation and imaging, and other uncontrolled factors. Here and throughout the paper, different channels in multichannel images are merged using RGB pseudo color scheme and the corresponding color labels for individual channels. The scale bar is 10 μm; N=6 cells were examined.