Fig. 9. Model of HSP47 and procollagen sorting at ER exit sites (ERES).
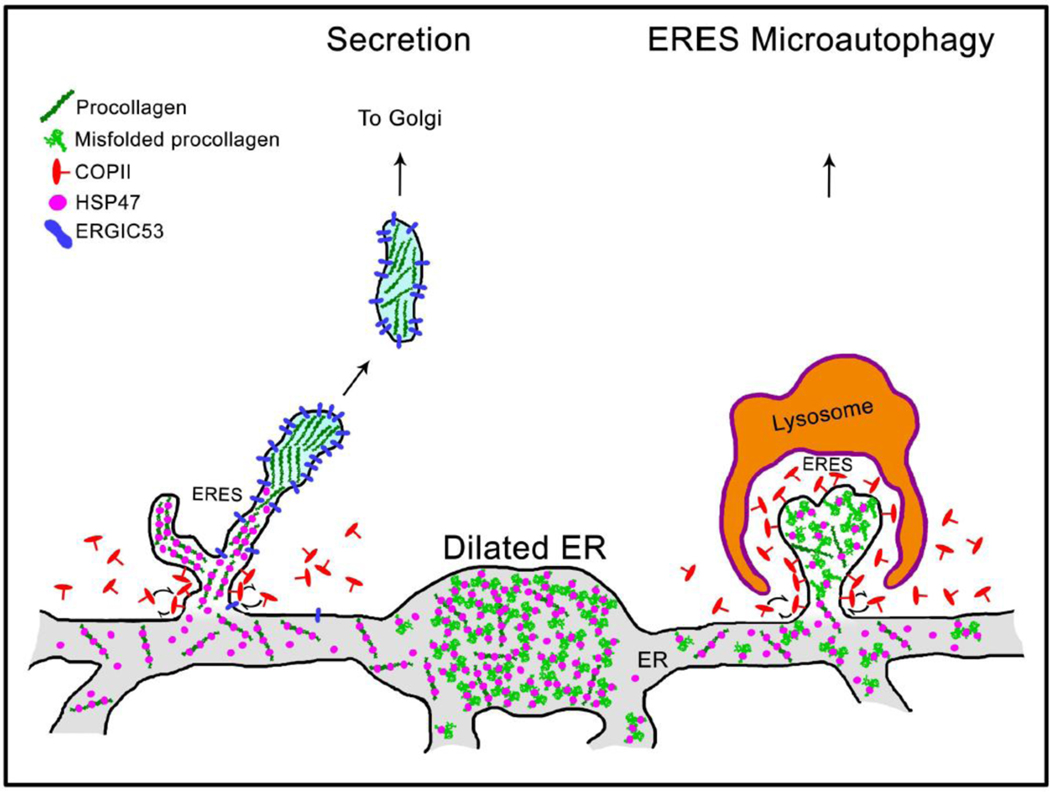
Grey structure at the bottom represents interconnected network of rough ER cisternae. Procollagen is loaded into ERES together with HSP47, as indicated by colocalization of procollagen, HSP47, and SEC23 (Fig. 8A, Movie 10). Maturation of procollagen transport intermediate precursors at distal ERES is accompanied by HSP47 release and accumulation of ERGIC53, since both ERGIC53 and HSP47 colocalize with procollagen at ERES and only ERGIC53 colocalizes with procollagen in Golgi-bound transport intermediates (Fig. 8, Movies 10–12). Unless its RDEL sequence is deleted or mutated, HSP47 is found in < 1±3% ERGIC53/procollagen transport intermediates, indicating that HSP47 is primarily returned to the ER from ERES (Supp. Fig. 7C). Only few escaping HSP47 molecules are likely to be captured by cis-Golgi KDEL/RDEL receptors and returned to the ER by retrograde trafficking. As reported before, ERESs containing misfolded procollagen and HSP47 might be engulfed by lysosomes and degraded (ERES microautophagy pathway) [37]. Under a microscope, procollagen ERESs (left), dilated ER regions (middle), and ERES microautophagy intermediates (right) appear as either small puncta or larger vesicle-like structures containing procollagen and HSP47 (Figs. 6 and 7, see also Figs. 1–7 in Ref. [37]). They accumulate upon inhibition of procollagen export from the ER by brefeldin A and are easy to confuse with transport vesicles, unless their motion and/or composition is imaged.
