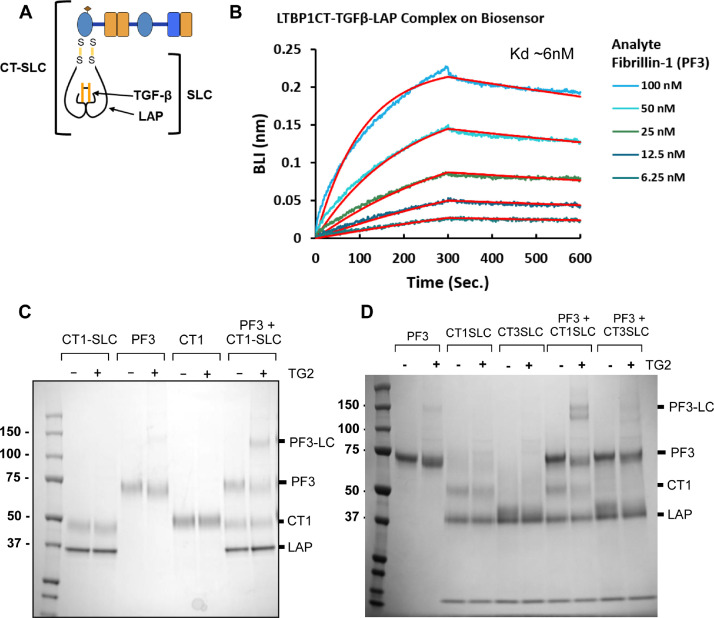
Fig. 3.
The large latent TGFβ complex can be cross-linked to fibrillin to form larger complexes. (A) Schematic diagram showing the CT1-SLC construct, where the LTBP1-CT region is co-expressed with SLC to form a truncated version of the large latent complex. (B) OctetRED Biolayer Interferometry (BLI) sensorgram showing the binding response detected between immobilized CT1-SLC and a range of concentrations (100-6.25 nM) of PF3. The binding affinity KD was determined by fitting to a 1:1 Langmuir binding model (red curves) and shows that fibrillin binds to LTBP1 with high affinity while part of the latent complex with TGFβ. Experiment was performed in duplicate and representative results are shown. (C) Reduced SDS-PAGE gel, stained with Coomassie Blue, showing the CT1-SLC, PF3, CT1 in the presence and absence of TG2. When PF3 and CT1-SLC are incubated in the presence of TG2, a larger species is formed indicating that cross-linking still occurs between PF3 and LTBP1 in the presence of latent TGFβ. (D) Reduced SDS-PAGE gel, stained with Coomassie Blue, showing the CT1-SLC or CT3-SLC with PF3 in the presence and absence of TG2 showing that cross-linking can also occur between PF3 and LTBP3 in the presence of latent TGFβ.