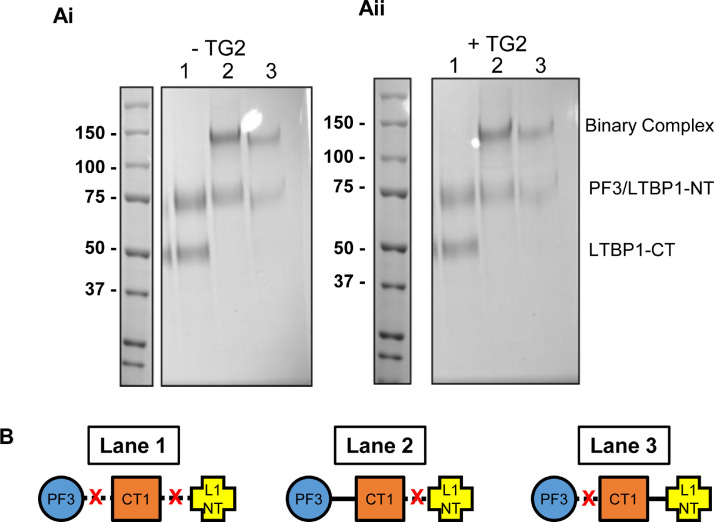
Fig. 4.
LTBP1 self-association competes with fibrillin interaction. (A) SDS-PAGE gels, stained with Coomassie Blue, assessing TG2 cross-linking of PF3, CT1 or the N-terminal region of LTBP1 (L1-NT) as either individual components or binary complexes. PF3, CT1 and L1-NT were mixed in the absence (i) or presence (ii) of TG2 (lane 1). In lane 2, PF3-CT1 binary complexes were mixed with L1-NT in the absence (i) or presence (ii) of TG2. Whereas in lane 3, L1NT-CT1 binary complexes were mixed with PF3 in the absence (i) or presence (ii) of TG2. In all cases no additional species, larger than the binary complexes, were formed as illustrated in (B), indicating that LTBP1 can either be involved in fibrillin cross-linking or LTBP1 self-association. Furthermore, no binary complexes were formed when all three components were mixed simultaneously (lane 1), indicating that interactions between the CT1 region and PF3 or L1-NT prevents TG2 cross-linking.