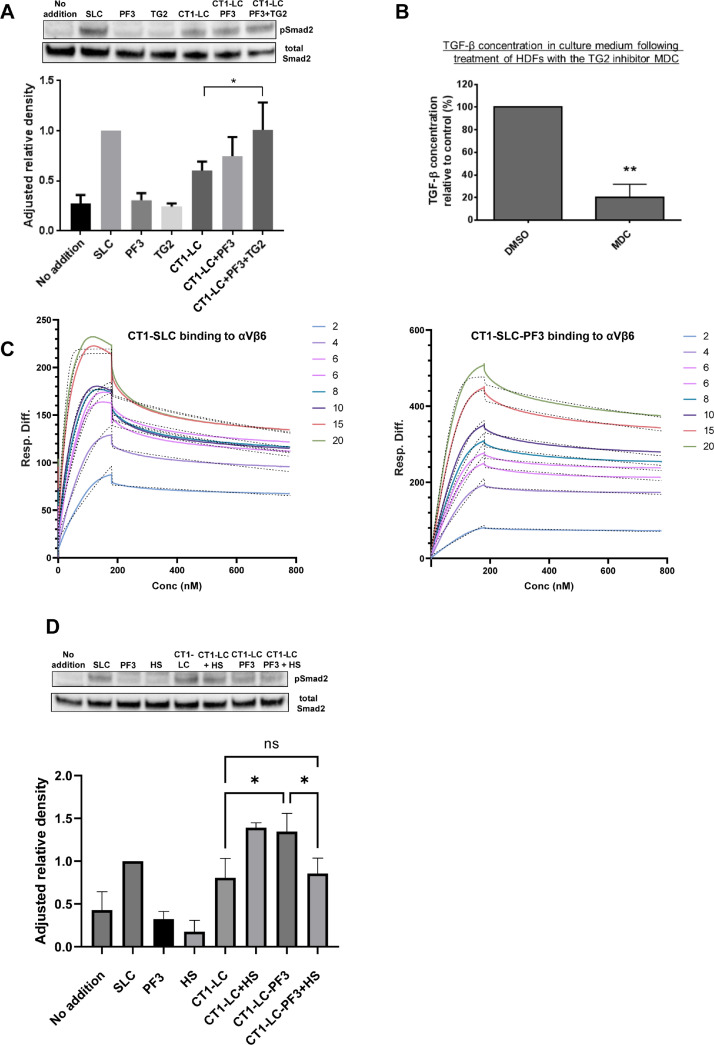
Fig. 6.
Cross-linking the latent TGFβ complex to fibrillin directs TGFβ to the cell surface for activation. (A) Western blotting to assess Smad2 phosphorylation compared to total Smad2 protein levels in HDFs treated with PF3, CT1-SLC, CT1-SLC plus PF3 or CT1-SLC-PF3 cross-linked complex. For each experiment, pSmad2 levels were analysed by densitometry and normalised to total Smad2 protein levels, with mean values for each treatment expressed as a proportion of SLC ± SEM. (B) HDFs were cultured for 5 days with DMSO (Control) or 100 μM MDC in DMSO. Media were analysed by ELISA for active TGFβ and shown as a percentage relative to the control. Results shown are representative of three independent experiments (n=3). ** indicates P < 0.01 from an unpaired T-test. (C) Surface plasmon resonance curves showing the binding response detected between either immobilised CT1-SLC or CT1-SLC-PF3 and integrin αVβ6. Proteins were immobilised via the Twin-Strep tag onto a Strep-Tactin XT surface and a range of concentrations (20-2 nM) of integrin αVβ6 used as the analyte. The binding affinity KD was determined by fitting to a 1:1 Langmuir binding model (dotted lines). Experiments were performed in triplicate and representative results are shown. (D) Western blotting to assess Smad2 phosphorylation and total Smad2 protein levels in HDFs treated with CT1-SLC or CT1-SLC-PF3 complex with or without addition of 5-molar excess of HS. For each experiment, pSmad2 levels were analysed by densitometry and normalised to total Smad2 protein levels, with mean values for each treatment expressed as a proportion of SLC ± SEM. For (A) and (D) results shown are representative of three independent experiments (n=3). * indicates P < 0.05 as determined by 1-way ANOVA with Tukey's post-hoc test.