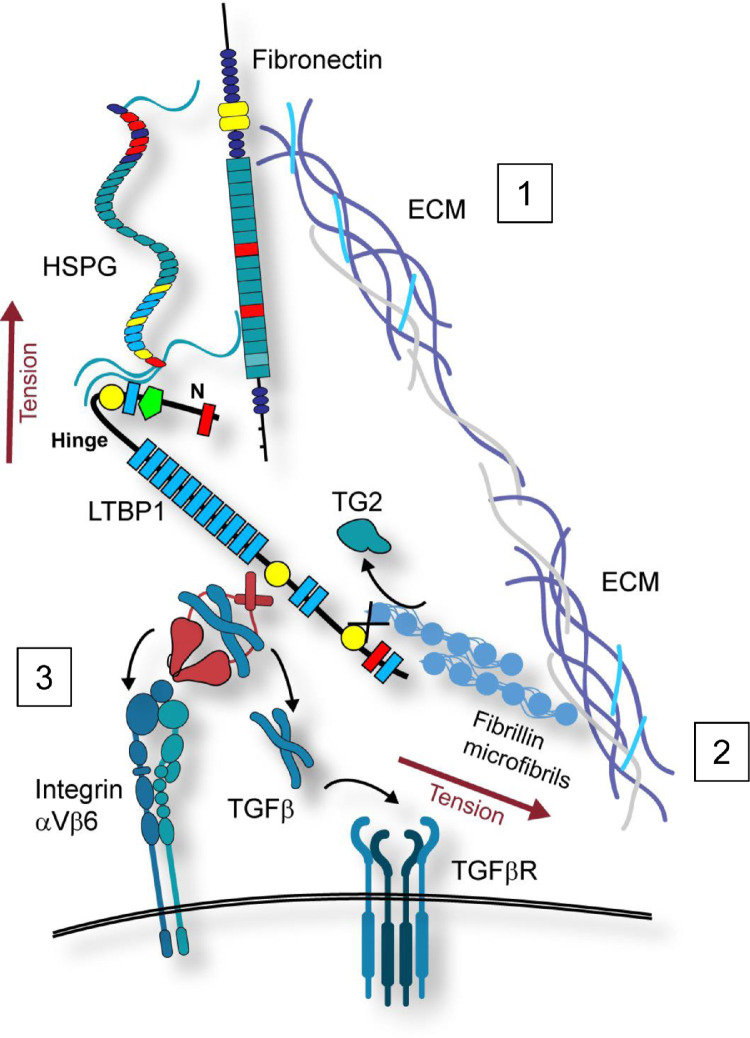
Fig. 7.
Model showing TG2 cross-linking of latent TGFβ complexes to fibrillin or fibronectin-matrices facilitates TGFβ activation. Cartoon showing how cross-linking the latent TGFβ complex to the matrix supports TGFβ activation. 1) Fibronectin and TG2 are required for activation of the LLC. The interaction with fibronectin which may be mediated through a heparan sulphate proteoglycan (HSPG), is thought to involve the LTBP1 hinge region as LLC constructs missing the hinge region are not activated and LTBP1 constructs lacking the N-terminal region are not incorporated into the matrix where it is thought that matrix targeting is needed for TGFβ activation. TG2 cross-linking increases TGFβ activation potentially by directing LLC complexes to the cell surface. This is thought to involve FN and HS interactions but fibronectin and LTBP1 are not directly cross-linked in vitro, suggesting an intermediary may be involved or conformational dependent interactions are required. 2) TG2 cross-linking of LTBP1 to fibrillin via the C-terminal region of LTBP1 provides another matrix anchor and supports TGFβ activation in the absence of the hinge region which explains why an antibody to the C-terminus of LTBP1 could block TGFβ activation. 3) Integrin binding to LAP (red) provides resistant forces between the cell surface receptor and matrix-tethered LLC which results in force activation of TGFβ leading to signalling via TGFβ receptors.