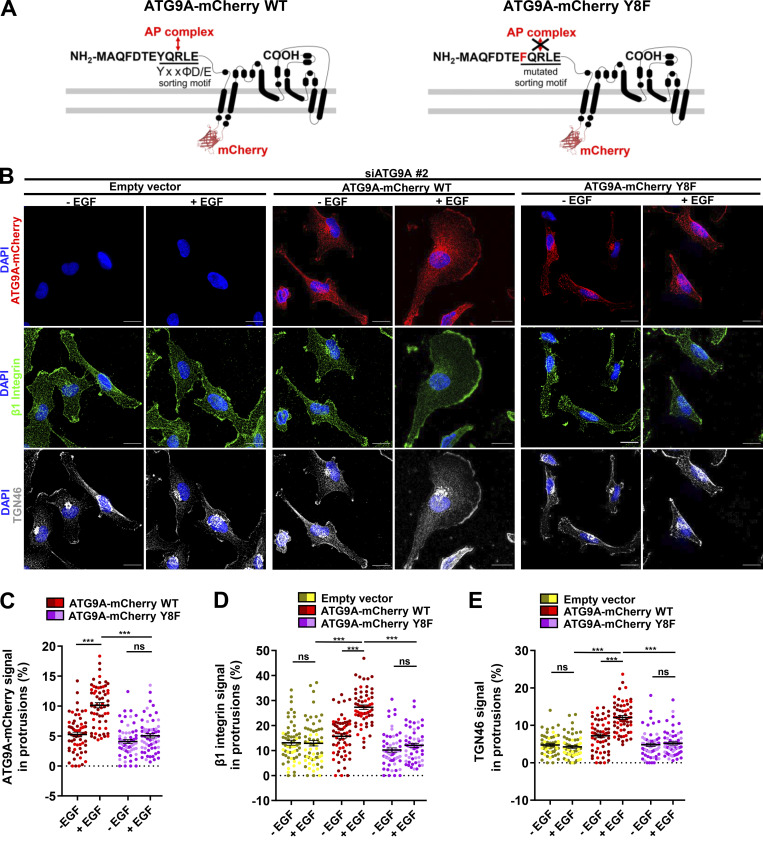
Figure 6.
ATG9A regulates delivery of TGN46 and β1 integrin to the leading edge through its N-terminal AP sorting signal. (A) Left: Scheme depicting the topology of the ATG9A-mCherry fusion protein and the canonical AP sorting signal (8YQRLE12) located in the N-terminal cytosolic domain. Right: Scheme depicting the mutant ATG9A-mCherry protein, for which replacement of the critical tyrosine residue at position 8 by a phenylalanine abolishes AP binding (Zhou et al., 2017; Mattera et al., 2017). (B–E) Rescue experiments with wild-type and mutant ATG9A-mCherry proteins. (B) After depletion of endogenous ATG9A using interfering RNA (siATG9A #2), U87 MG cells were transfected with an empty vector, a vector encoding wild-type ATG9A-mCherry, or a vector encoding mutant ATG9A-mCherry Y8F. Transfected cells were starved (30 min) in serum-free medium and incubated (30 min) with or without EGF (50 ng/ml), as indicated. Cells were fixed and labeled for β1 integrin (green), TGN46 (white), and nuclei (DAPI, blue). Scale bar, 20 µm. (C) Quantification from images shown in B of the ATG9A-mCherry (wild-type or mutant) signal located in protrusions. (D) Quantification from images shown in B of the β1 integrin signal located in protrusions. (E) Quantification from images shown in B of the TGN46 signal located in protrusions. Data represent means and SEM (n = 60–63 cells per group; cells from independent experiments were color-coded). Statistical significance was evaluated using one-way ANOVA followed by Tukey post hoc test. ***, P < 0.001.