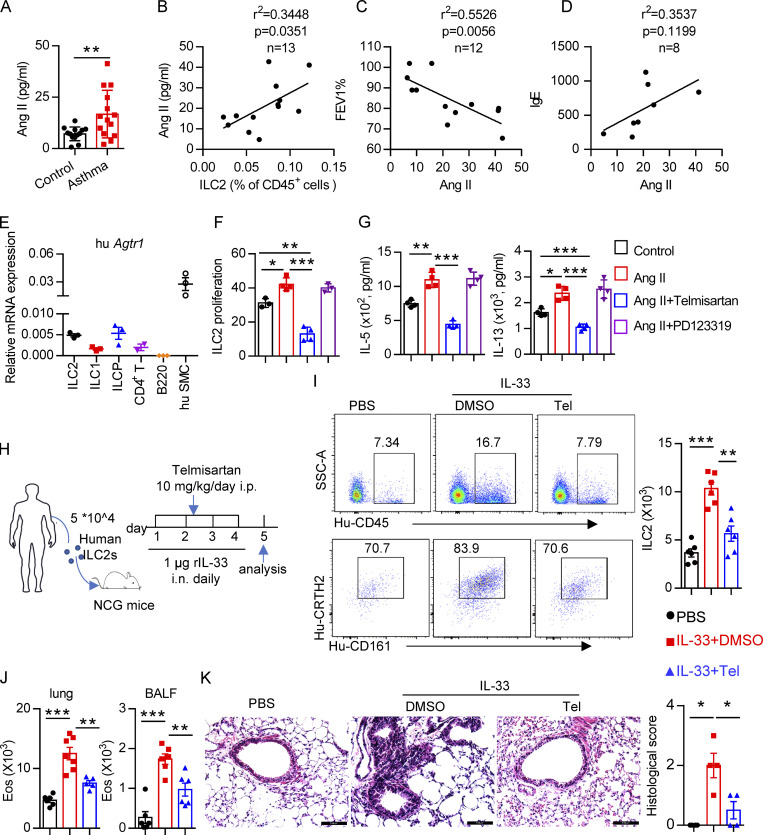
Figure 8.
Clinical relevance of Ang II in patients with asthma. (A) Plasma Ang II in healthy controls and asthmatic patients; control, n = 13; asthma, n = 14. (B–D) Correlation between Ang II concentration and the percentage of circulating ILC2s, n = 13 (B), FEV1%, n = 12 (C), and IgE, n = 8 (D), in asthmatic patients. (E) mRNA expression of AT1a in the indicated immune cells from PBMCs of healthy volunteers. n = 3/group, and data are repeated two times. (F and G) Purified human ILC2s were cultured in medium containing 20 ng/ml IL-2, IL-7, and IL-33 for 3 d, in the presence of the indicated treatments including Ang II, Ang II plus telmisartan, and Ang II plus PD123319. Proliferation of ILC2s was determined by CFSE staining (F) and amount of IL-5 and IL-13 in culture supernatants (G). Data are from two independent experiments; n = 4/group. (H–K) Human ILC2s (5 × 104) were intravenously transferred into NCG mice, followed by i.n. challenge with rh-IL-33 (1 µg/d) or PBS. Recipient mice were i.p. administered telmisartan or DMSO for 4 consecutive days. Mice were sacrificed 24 h after the last challenge. (H) Experimental strategy. (I) Proportions and absolute numbers of human ILC2s in lung. (J) Absolute cell counts of eosinophils in lung and BALF of recipients. (K) H&E staining of lung sections and quantitated histological scoring (scale bar, 100 µm). (H–K) Data are representative of two independent experiments; n = 3 or 4/group. Graphical data show mean ± SEM; unpaired t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Eos, eosinophil; hu SMC, human smooth muscle cell; SSC-A, side scatter area; Tel, telmisartan.