Fig. 2 |. Conserved nutrient-sensing response pathways in worms, flies and mammals.
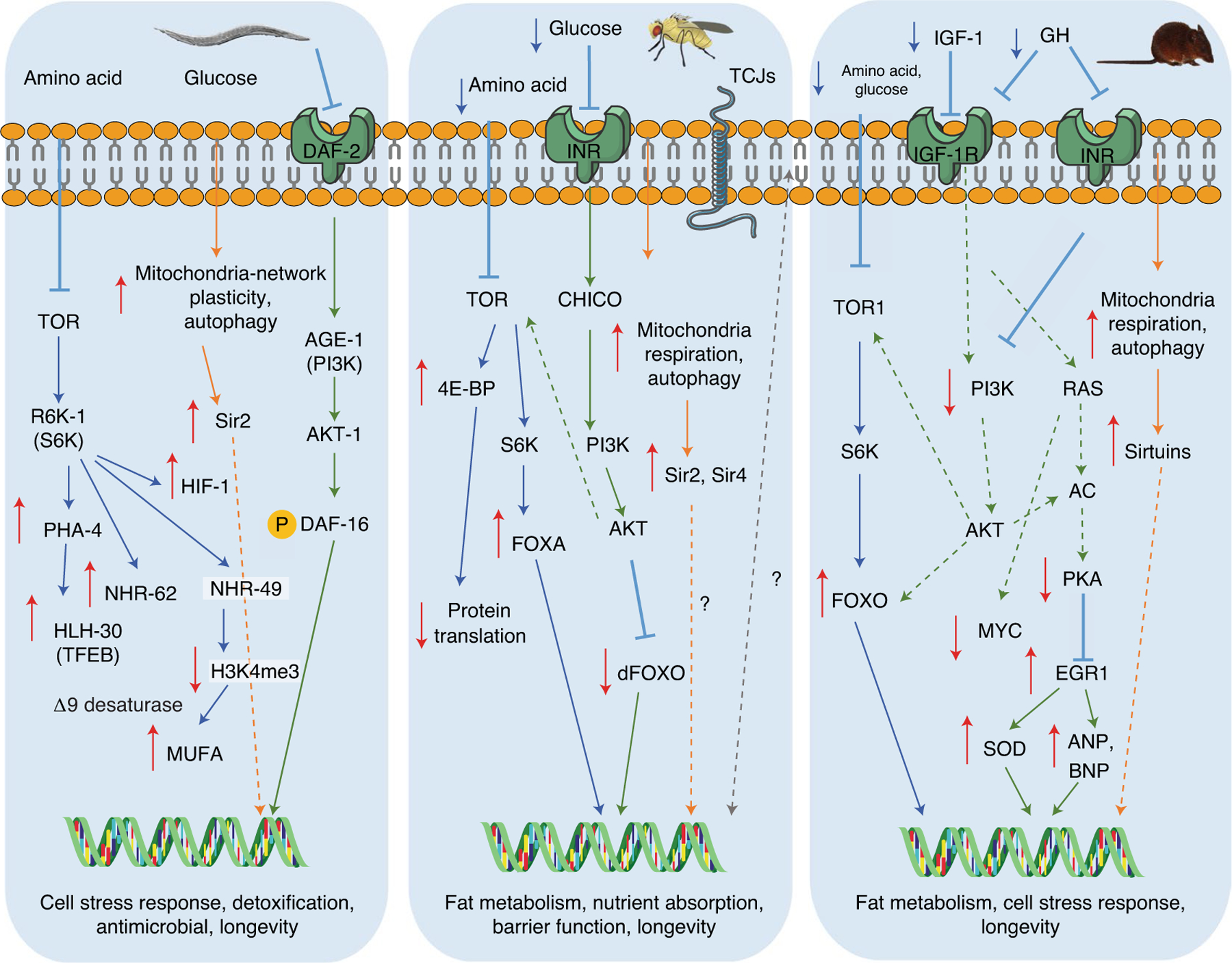
This model summarizes the conserved nutrient-sensing pathways that regulate longevity and stress-response mechanisms in different model organisms1. Fasting or calorie restriction reduces the activity of amino acids and glucose signaling pathways through membrane receptors by reducing circulating ligands such as growth factors like mammalian IGF-1. The fasting-inhibited TOR–S6K pathway (labeled in blue) promotes the expression of nuclear transcription factors such as the hypoxia-inducible factor-1 (HIF-1), the FOXA ortholog PHA-4, the nuclear hormone receptors NHR-62 and NHR-49 and the TFEB ortholog HLH-30 (worms), the FOXA nuclear factor (flies) and the increase of the FOXO nuclear factor (mammals). These transcription factors commonly activate antiaging systems and processes, including autophagy and ribosomal biogenesis, stress response and cellular-protection genes, including antioxidant SODs. The RAS–AC–PKA pathway (labeled in green) is also partially conserved between species. Similar to what was observed in yeast, glucose in certain mammalian cells can signal through the PKA pathway and the transcription factor EGR1, the mammalian ortholog of Msn2/Msn4 in yeast. In worms, flies and mice, downregulation of TOR–S6K signaling has conserved proaging effects1. The fasting-dependent effects on longevity in different organisms may also involve sirtuin pathway activation (labeled in orange), the increase in mitochondria respiration and the activation of autophagy39,40. Also, fasting in flies delays the disruption of tricellular junctions (TCJs), which is linked to improved intestinal barrier integrity and therefore longevity (labeled in gray)148.
