Figure 5.
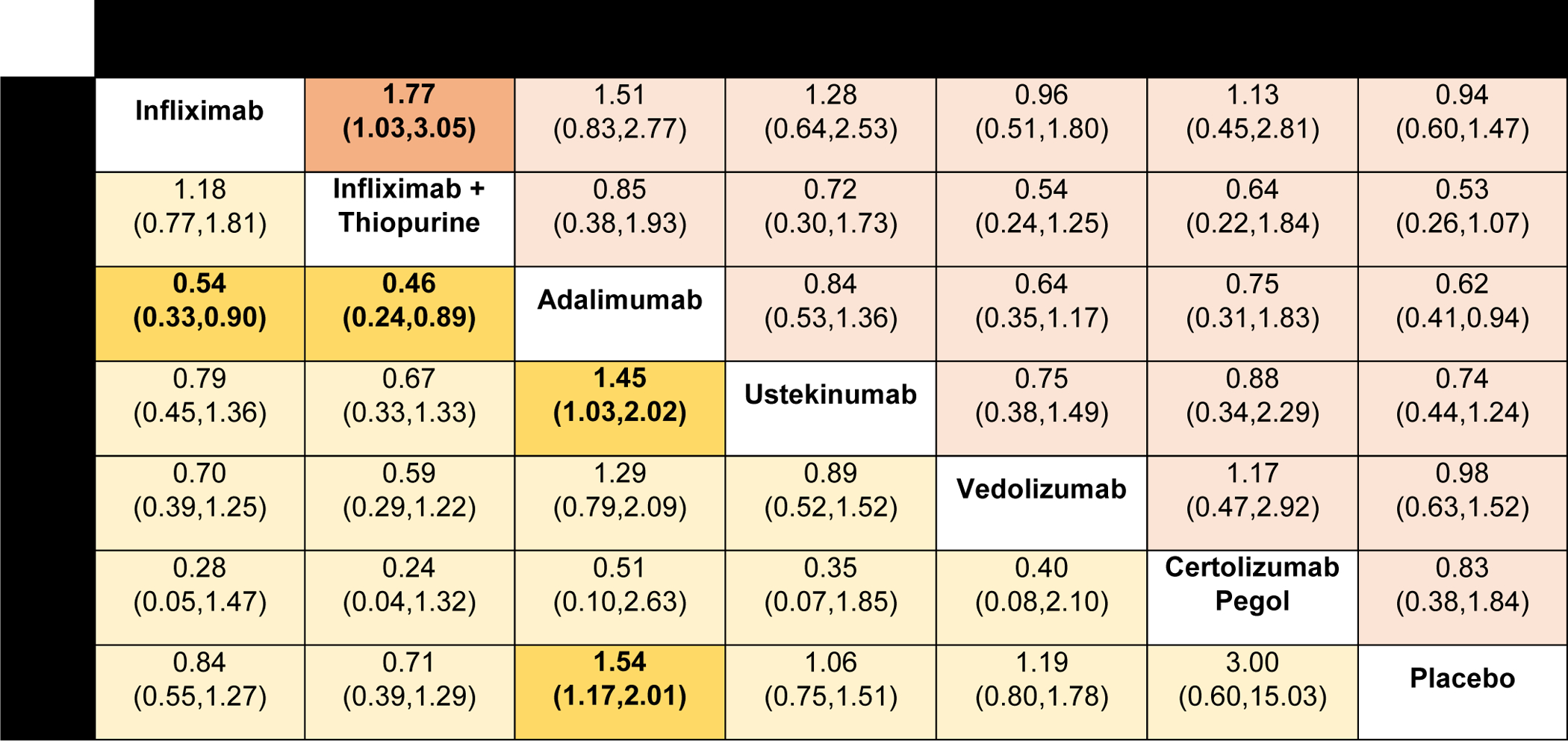
Comparative safety of biologic agents for risk of serious adverse events and any infections in patients with moderate-to-severe Crohn’s disease, using network meta-analysis, expressed as odds ratios with 95% confidence intervals. Comparisons read from left-to-right; odds ratios for comparisons in the cell in common between column- and row-defining treatment. For serious adverse events, odds ratio <1 favors row-defining treatment. For risk of infections, odds ratio <1 favors column-defining treatment.
