Abstract
Temporal lobe epilepsy (TLE) is a network disorder with a high incidence of memory impairment. Memory processing ability highly depends on the dynamic coordination between distinct modules within the hippocampal network. Here, we investigate the relationship between memory phenotypes and modular alterations of dynamic functional connectivity (FC) in the hippocampal network in TLE patients. Then, 31 healthy controls and 66 TLE patients with hippocampal sclerosis were recruited. The patients were classified into memory‐intact (MI, 35 cases) group and memory‐deficit (MD, 31 cases) group, each based on individual's Wechsler Memory Scale‐Revised score. The sliding‐windows approach and graph theory analysis were used to analyze the hippocampal network based on resting state functional magnetic resonance imaging. Temporal properties and modular metrics were calculated. Two discrete and switchable states were revealed: a high modularized state (State I) and a low modularized state (State II), which corresponded to either anterior or posterior hippocampal network dominated pattern. TLE was prone to drive less State I but more State II, and the tendency was more obvious in TLE‐MD. Additionally, TLE‐MD showed more widespread alterations of modular properties compared with TLE‐MI across two states. Furthermore, the dynamic modularity features had unique superiority in discriminating TLE‐MD from TLE‐MI. These findings demonstrated that state transitions and modular function of dissociable hippocampal networks were altered in TLE and more importantly, they could reflect different memory phenotypes. The trend revealed potential values of dynamic FC in elucidating the mechanism underlying memory impairments in TLE.
Keywords: chronic epilepsy, dynamics, hippocampal sclerosis, memory disorder, MRI, neuroimaging
The present research provides evidence that the modular organization of the hippocampal network fluctuates over time, presenting either anterior or posterior hippocampal network dominated states. We highlight that functional differences between Temporal lobe epilepsy (TLE) with and without memory impairment can be observed in modular alternations of the hippocampal network, which indicated that the dynamic functional alternations of the hippocampal network are more sensitive in reflecting memory impairment in TLE. Our findings introduced a new perspective of understanding the dynamic functional connectivity of memory network in different memory status in TLE, which could potentially facilitate the diagnosis and treatment strategies.
1. INTRODUCTION
Memory disorder is a common cognitive comorbidity of temporal lobe epilepsy (TLE). Around 50% of the TLE patients suffer greatly from severe memory deficits, the rests remains relatively intact (Helmstaedter & Elger, 2009; Thompson & Corcoran, 1992). In the 1960s, the researchers considered that memory impairment may be related to the damaged integrity of the hippocampus in TLE (Milner, 1968). Since the early 1990s, there has been a shift toward the thinking that the degree of memory impairment is closely associated with the extent of abnormalities in the hippocampal memory circuits (Bell, Lin, Seidenberg, & Hermann, 2011; Saling, 2009; Squire & Zola‐Morgan, 1991). Importantly, the heterogeneity of memory performance with TLE may indicate different cognitive phenotypes relate to the whole brain network changes (Dabbs, Jones, Seidenberg, & Hermann, 2009; Kaestner et al., 2019; Rayner, Tailby, Jackson, & Wilson, 2019; Reyes et al., 2019). Cognitive phenotypes have the potential to provide marks for treatment and prognosis. However, it is not well known that whether the “memory‐deficits” (MD) and “memory‐intact” (MI) phenotypes within TLE have unique patterns of the hippocampal “network” characteristics. Thus, further elucidating the hippocampal network underlying the memory phenotypes may provide individualized strategy for TLE management.
The hippocampal network consists of regions in medial temporal lobe (MTL) and extra‐MTL, some of which are believed to be involved in memory processes in TLE. Previous studies using task based functional magnetic resonance imaging (fMRI) techniques in TLE demonstrated that the aberrant functional coupling and activation in the MTL, including the hippocampus, perirhinal cortex (PRC), and parahippocampal cortex (PHC), were closely related to the episodic memory declines (Doucet, Osipowicz, Sharan, Sperling, & Tracy, 2013; Figueiredo et al., 2008; Powell et al., 2007; Vannest, Szaflarski, Privitera, Schefft, & Holland, 2008). Moreover, anterior extra‐MTL including regions like orbitofrontal cortex (OFC), amygdala (AMY), and anterior fusiform gyrus (FUS) and posterior extra‐MTL including regions like posterior cingulate cortex (PCC), retrosplenial cortex (RSC), precuneus (PREC) anterior inferior temporal cortex (ITC), and angular gyrus (ANG) were reported to be abnormal activation in TLE across different memory process (Addis, Moscovitch, & McAndrews, 2007; Bonelli et al., 2009; Guedj et al., 2011; Hill, King, Lega, & Rugg, 2020; Sidhu et al., 2013). Further studies of large‐scale network analysis focusing on the hippocampal network indicated widespread abnormalities of functional connectivity (FC) as well as nodal topological properties, which contribute greatly to identifying memory abilities in TLE (Bettus et al., 2009; McCormick et al., 2014; Roger, Pichat, Torlay, & David, 2020). However, most of previous studies did not consider the important aspect of the functional diversity in this network, as the hippocampus was assessed as a unitary structure.
Recent evidence considered that there are dissociable hippocampal networks along the anterior–posterior axis of the hippocampus (Poppenk & Moscovitch, 2011; Ranganath & Ritchey, 2012; Voets et al., 2014). More specifically, the modularity feature was found in the hippocampal network, suggesting that anterior MTL (e.g., anterior hippocampus and PRC) and posterior MTL (e.g., posterior hippocampus and PHC) are connected with extra‐MTL modules through different neuroanatomical pathways. The anterior and posterior MTL implicated in sematic knowledge presentation and scenes/spatial layouts respectively, and their interplay with extra‐MTL modules serves as integration for memory system (Ritchey, Libby, & Ranganath, 2015). Our previous study demonstrated that alteration in this conceptual framework contributes to revealing the changes of diverse memory systems in TLE (Li et al., 2017).
The brain network can be characterized by the principles of graph theory analysis, in particular the characteristic of modularity. Typically, modularity can be described as the brain network with a given module partition, presenting spatially separated modules with densely intramodular connections and sparsely intermodular connections (Sporns & Betzel, 2016). In addition, the interactions between specialized brain modules with fluctuating over time were observed by dynamic FC analysis, presenting reoccurring patterns of brain states (Allen et al., 2014; Bosman, Lansink, & Pennartz, 2014; Chang & Glover, 2010; Gonzalez‐Castillo et al., 2015). Modular segregation and integration are two basic brain patterns (Friston, 2002), which were reported within the hippocampal network dominating in episodic memory encoding and retrieval, respectively (Cooper & Ritchey, 2019). Investigating modularity features of the hippocampal network and their dynamic fluctuations may provide important evidence for understanding the mechanism of memory impairment in TLE.
Herein, we hypothesized that TLE patients with different memory phenotypes may have distinct functional modular patterns of the hippocampal network during resting. In this study, we used resting‐state fMRI and a combination method of graph theory analysis and sliding‐window approaches to test this hypothesis. Our aim involves two aspects: (a) whether the hippocampal network has integrated and segregated states during resting and (b) whether the temporal properties of the dynamic hippocampal network can predict memory phenotypes in TLE.
2. MATERIAL AND METHODS
2.1. Participants
This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. (Study No. 2014‐151), and written informed consent was obtained from all participants. Then, 79 right‐handed subjects, including 66 TLE patients with unilateral hippocampal sclerosis (HS) (left, 35; right, 31) and 31 healthy controls (HC), were consecutively enrolled from April 2014 to January 2020. All patients were referred from the comprehensive epilepsy center in our hospital. Each patient was diagnosed based on the detailed history, neurological examination, video‐EEG recordings, and MRI findings. The inclusion criteria included: (a) unilateral HS without other abnormalities on MRI such as cerebral trauma, tumors, vascular malformations, or malformations of cortical development and (b) electroclinical features suggesting ipsilateral TLE. Patients were excluded if they met any of the following criteria: (a) a history of brain trauma or surgery, (b) evidence of an infectious origin, (c) a severe psychiatric disorder, or (d) bilateral TLE. Clinical and imaging data including detailed medical history, scalp video electroencephalogram, routine epileptic MRI, high resolution hippocampal T2WI images, and neuropsychological examination were obtained. HC without neurological or psychiatric disorders were recruited.
2.2. Neuropsychological measurements
Immediate retrieval tests were performed by the Wechsler Memory Scale‐Revised, Chinese version. The scores of the verbal paired associates and logical memory tests were added together as verbal memory scores, as well as the scores of the figure memory, recognition and visual reproduction tests were added together as nonverbal memory scores. The total scores of verbal and nonverbal memory were converted into z‐scores separately. Impairment was defined as memory z‐scores at least 1.5 SDs below the mean HC data. Taking into account the limited sample size, two groups of memory phenotypes would be appropriate for statistical analysis in this present study. In our study, all patients completed each memory subtest. Patients were determined to be impaired in the memory domain if verbal memory or nonverbal memory z‐scores fell within the impaired range. Then, 66 patients were classified into 2 groups, within which 35 as MI and 31 as MD (verbal memory impairment: 17, nonverbal memory impairment: 3, both verbal and nonverbal memory impairment: 11).
2.3. MRI acquisition
Structural MRI and resting state fMRI data were acquired using a 3 T Discovery MR750 (GE Healthcare) scanner with an eight‐channel head coil in the Second Affiliated Hospital, Zhejiang University School of Medicine. All patients were scanned during the interictal period (at least 3 days after the latest focal seizure and at least 7 days after the latest focal to bilateral generalized tonic–clonic seizure). All subjects were asked to keep their eyes closed, let their mind relaxed during scans. T1 weight structural data were acquired using a high‐resolution three‐dimensional brain volume (3D‐BRAVO) imaging sequence with the following parameters: repetition time (TR)/echo time = 8.2/3.2 ms, inversion time = 450 ms, flip angle = 12°, matrix = 256 × 256, voxel size = 0.47 × 0.47 × 1 mm3 without slice gap, and 206 slices. Resting‐state data were obtained using a gradient‐echo echo‐planar imaging sequence (TR/echo time = 2000/30 ms, flip angle = 77°, matrix = 64 × 64, voxel size = 3.75 × 3.75 × 4 mm3 with no gap, number of slices = 38), for a total of 180 TRs (6 min) or 195 TRs (6.5 min) per scan.
2.4. Analysis
2.4.1. Functional image preprocessing
Resting‐state fMRI data were preprocessed using SPM 12 (www.fil.ion.ucl.ac.uk/spm/) and DPABI software (http://rfmri.org/dpabi). To maintain data consistency, we selected the first 180 brain volumes of each subject for the following analysis. The first 10 scans were removed to stabilize the MRI baseline signal. The following steps include slice‐time correction, realignment, spatial normalization with resampling to 3‐mm isotropic voxels through T1 weight structural images, linear detrending, regression of nuisance covariates (Friston‐24 head motion parameters, white matter, cerebrospinal fluid signals), and temporal filtering (0.01–0.1 Hz). Smoothing was unapplied for avoiding extraction of averaged component with mixed signals that belong to outside ROIs.
2.5. Network construction
ROIs of MTL and extra‐MTL were defined to construct a hippocampal network. These ROIs comprise the MTL (anterior nodes: anterior hippocampus and PRC); posterior nodes: posterior hippocampus and PHC) and the extra‐MTL (anterior nodes: AMYG, anterior FUS, anterior ITC, OFC; posterior nodes: RSC, PCC, PREC, and ANG). The anterior and posterior hippocampus masks were created using voxel‐wise correlation analysis. Details of the parcellation of the hippocampus steps are described in our previous study (Xu, Guan, Li, Xu, & Zhang, 2019). The PHC and PRC masks were extracted from an MTL atlas (https://neurovault.org/collections/3731/). The other ROIs were obtained from the Harvard‐Oxford Structural Atlas (https://www.fmrib.ox.ac.uk/fsl/data/atlas-description.html). In total, 24 target masks were assigned to ipsilateral and contralateral hemisphere (12 for each hemisphere) subsequently used for the analyses. By generating pairwise Pearson coefficients between each pair of mean ROI time series and proceeding to use Fisher z‐transformation (Fz) to stabilize the distribution of Pearson's correlation, we obtained a hippocampal network 24 × 24 Fz weighted matrix for each subject. We flipped the corresponding regions of the right hemisphere to the left hemisphere in right TLE‐HS patients and proportionate HC for the following analysis.
2.6. Dynamic functional analysis
Dynamic functional analysis was performed using DynamicBC toolbox (http://restfmri.net/forum/DynamicBC) with the sliding‐window approach and k‐mean clustering method (see Supporting Information Figure S1 in Appendix S1). First, to capture the hippocampal network time‐varying correlations, we segmented the whole time series into a fixed window with a width of 20 TRs. This setting has demonstrated good balance between the estimation of dynamic connectivity and statistical tests, owing to no suppression of blood oxygenation level‐dependent frequency beyond 0.01 Hz (Zalesky & Breakspear, 2015). With a step‐wise slide of 1 TR along the 170‐TR length scan, we constructed 150 successive 24 × 24 matrices for each subject. The resulting correlation matrices were converted to z‐values with Fz.
Second, we used the K‐means clustering algorithm to cluster dynamic FC patterns across control and patients together (Lloyd, 1982). This clustering algorithm was repeated 100 times to weaken the impact of local minima (Pascual‐Marqui, Michel, & Lehmann, 1995). The correlation distance function was used to measure similarity between FC matrices of different windows since the correlation distance metric is more sensitive to the FC pattern irrespective of magnitude (Damaraju et al., 2014). A K of 2 were obtained using three recognized evaluation indexes (Silhouette, Calinski‐Harabasz, and Davies‐Bouldin) in a search range of K from 2 to 10 (see Supporting Information Figure S2 in Appendix S1). Afterward, the resulting optimal clusters were indicated as two corresponding cluster centroids, which were regarded as two dynamic FC states across subjects and windows. Using these subjects state vectors (a vector indicating which state each time point is assigned to), we assess the temporal variability of dynamic FC: (a) fractional windows, means the occupancy of each state, which is calculated as a ratio between the number of windows assigned to each state and the number of total time windows; (b) mean dwell time, denotes the window length of the subject staying in certain state, which is calculated as an average of the consecutive number of windows assigned to certain state before changing to the other state; and (c) number of transitions, calculated as the number of windows switching from one state to the other state.
2.7. Static and dynamic modular analysis
A predefined module parcellation was applied for the modular analysis of the hippocampal functional networks, including anterior MTL module, posterior MTL module, anterior extra‐MTL module and posterior extra‐MTL module (see Supporting Information Figure S1 for more detail). This predefined module partition provides an unbiased sample‐independent reference for group statistical comparisons and keep a consistent framework for the subsequent modular interaction analysis across static and dynamic states.
We investigated the static modular properties of this hippocampal network in each participant: (a) global modularity value, reflecting the degree to which a set of nodes are operating as distinct networks based on their covariation in activity (see Supporting Information “Global modularity analysis” section in Appendix S1 for more detail) and (b) intramodular and intermodular connectivity, calculated as the mean node‐to‐node connectivity of all nodes within a module and the mean node‐to‐node connectivity for each pair of nodes between distinct modules.
For dynamic functional modular analysis, 151 time‐varying functional correlation matrices for each subject were transformed into the corresponding Fz weight matrices. Only positive correlations of each windowed correlation matrix were selected to generate dynamic weighted functional networks for each subject. Then, a predefined module partition was applied for calculating intrasubject modular properties of dynamic weighed functional networks. Each window of intrasubject dynamic modular properties was labeled by individual's state vectors described above. For each subject, the modular properties which belong to the same state were averaged to present the state‐specific modular pattern.
2.8. Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of the demographic and clinical characteristics. Group differences in age, education, age at onset, epilepsy duration, seizure frequency, number of antiepileptic medications, and memory scores were determined using independent t test or Mann–Whitney test. Gender distribution, carbamazepine, and topiramate load between groups were compared using the χ2 test. These analyses were completed using IBM SPSS version 19.
For the modular metrics and temporal properties, the F real was initially calculated in three groups. Then, the permutation test was performed for ANOVA. We randomly reallocated all subjects to three groups with the same sample size to obtain the F surrogate (10,000 permutations). The observed proportions of the F surrogate, which was greater than F real in the null distribution, were detected for estimating the p value. The post hoc analysis was conducted by adjusting on the multiple‐group level with Bonferroni correction. After multiple‐group level correction, the modular metrics of each state and temporal properties were conducted by adjusting on multiple‐metrics level FDR correction separately. Modular metrics of each state included 11 measurements (e.g., global modularity, intramodular connections, intermodular connections). Temporal properties included four measurements (e.g., fractional windows, mean dwell time, number of transitions).
2.9. Validation
Although a window size in the range of 30–60 s has been widely used to capture the real variations in the brain connectivity (Allen et al., 2014; Hutchison, Womelsdorf, Allen, et al., 2013; Hutchison, Womelsdorf, Gati, Everling, & Menon, 2013), a mismatch between cut‐off frequencies for filtering and the choice of the window length may cause a spurious variability of dynamic FC (Leonardi & Van De Ville, 2015). To validate the stability and robustness of the results, we repeat our analysis using a sliding window approach with different window sizes (15 TRs, 30 TRs, 40 TRs, 50 TRs).
To further investigate whether the temporal properties and modular metric differences between two patient groups depended on the side of the epileptogenic zone, the Scheirer–Ray–Hare test was used for 2 (TLE‐MI vs. TLE‐MD) × 2 (left TLE‐HS vs. right TLE‐HS) factorial ANOVA.
To validate the robustness of dynamic network properties at the individual level, we first performed logistic regression with a backward stepwise selection to identify the most important variables that can discriminate TLE‐HS patients between TLE‐MI and TLE‐MD. The most important variables between two patient groups were selected as input for the logistic regression model. From the resulting logistic model, we conducted the receiver operating characteristic (ROC) analysis to obtain the compositive scores. The best discriminative result was identified by the Youden index.
The logistic regression was operated to determine whether dynamic FC variables are superior to hippocampal volumetric variables for classification of TLE‐MD versus TLE‐MI.
The ratio of each side hippocampal volume (HPV) divided by total intracranial volume (TIV) was estimated by individual’s structural MRI and compared between groups (see Supporting Information “Hippocampal volume analysis” section in Appendix S1 for more detail). The model performance was compared by the area under the curve (AUC) for models with significant hippocampal volumetric information only to models that also include the significant dynamic FC variables. The 95% confidence interval test with 10,000 bootstrapped samples was computed for assessing the differences between model AUCs. A superior model was identified if the 95% confidence interval for AUC was above that of the model to be compared.
3. RESULTS
3.1. Demographic profiles and memory function
There was no difference in age or gender between TLE‐HS patients and the HC group. However, the education level was lower in TLE‐HS patients (p < .05). No significant differences in age, gender, duration of epilepsy, age at seizure onset, seizure frequency, number of antiepileptic drugs, or lateralization of the epileptogenic zone were observed between TLE‐MI and TLE‐MD group. Compared to TLE‐MD, TLE‐MI had more years of education (p < .005). The numbers of carbamazepine (p = .159) and topiramate (p = 0.335) load also did not significantly differ between TLE‐MD and TLE‐MI. Obviously, verbal memory (p < .0001) and nonverbal memory (p < .01) were significantly impaired in the TLE‐HS patients compared to HC. Moreover, the TLE‐MD group presented lower verbal and nonverbal memory scores than the TLE‐MI group (both p < .0001). In the TLE‐MD group, 28 patients (left, 18; right, 10) showed verbal memory impairment, and 14 patients (left, 9; right, 5) showed nonverbal memory impairment. Detailed information is shown in Table 1.
TABLE 1.
Summary of demographic and clinical data
| Item | TLE | HC | p‐Value |
|---|---|---|---|
| Number (F/M) | 66 (38/28) | 31 (15/16) | .529 a |
| Age | 30.43 (9.92) | 33.87 (10.03) | .117 b |
| Education | 10.98 (3.19) | 12.66 (4.29) | .042 c |
| Verbal memory z‐score | −1.27 (1.47) | 0 | <.0001 c |
| Nonverbal memory z‐score | −0.80 (0.99) | 0 | .001 c |
| TLE‐MD | TLE‐MI | ||
| Number (F/M) | 31 (14/17) | 35 (24/11) | .054 a |
| Age | 32.84 (10.53) | 28.31 (8.97) | .067 b |
| Education | 9.71 (2.93) | 12.11 (3.02) | .002 c |
| Side, L/R | 19/12 | 17/18 | .300 a |
| Age at onset | 17.06 (10.47) | 15.00 (8.30) | .384 b |
| Duration | 16.52 (10.06) | 13.80 (8.30) | .266 b |
| Seizure frequency per month | 9.24 (22.94) | 13.61 (31.17) | .827 c |
| Numbers of AED | 2.19 (0.79) | 2.2 (0.90) | .847 c |
| Patients on carbamazepine | 15 (45.2%) | 11 (31.4%) | .159 a |
| Patients on topiramate | 3 (3.2%) | 1 (5.7%) | .335 d |
| Verbal memory z‐score | −2.42 (1.02) | −0.18 (0.84) | <.0001 c |
| Nonverbal memory z‐score | −1.37 (0.92) | −0.22 (0.72) | <.0001 c |
Note: SDs are presented in parentheses.
Abbreviations: AED, antiepileptic drug; MD, memory deficit; MI, memory intact; TLE, temporal lobe epilepsy.
p‐Value was obtained by Pearson chi‐square test.
p‐Value were obtained by Student's t test.
p‐Value were obtained by Mann–Whitney test.
p‐Value were obtained by Fisher exact test.
3.2. Two connectivity states of the hippocampal network
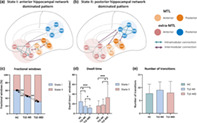
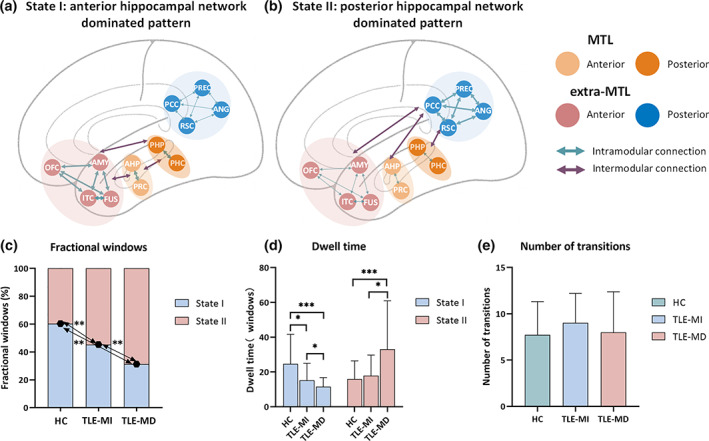
The dynamic FC was divided into two clusters that represent the transitional hippocampal network states. Figure 1 displays the corresponding visualized connectivity patterns (centroids of clusters) of the two common FC states: a highly modularized state (State I); and a lowly modularized state (State II).
FIGURE 1.
Functional connectivity state results. The centroid of group‐specific cluster for each state, averaged across subject‐specific median cluster centroids of each group. Respective percentages of occurrences for States I and II: 60.35 and 39.65% in the healthy controls (HC), 45.33 and 54.67% in temporal lobe epilepsy (TLE) with memory intact (MI), 31.32 and 68.68% in TLE with memory deficit (MD). The mean global modularity of State I was higher than that of State II in each group by using paired permutation tests. Each dot represents an individual mean global modularity score. ***p < .001; error bars represent SDs. The hippocampal network is divided into anterior medial temporal lobe (MTL), posterior MTL, anterior extra‐MTL and posterior extra‐MTL. AMYG, amygdala; ANG, angular gyrus; Ant, anterior; c, contralateral; FUS, anterior fusiform gyrus; ITC, anterior inferior temporal cortex; l, ipsilateral; OFC, lateral orbitofrontal cortex; PCC, posterior cingulate cortex; PHC, parahippocampal cortex; Pos, posterior; PRC, perirhinal cortex; PREC, precuneus; RSC, retrosplenial cortex
3.3. Temporal properties of FC states
A significant difference on fractional windows was identified among the three groups (F = 15.5766, p < .001). In HC, the fractional windows in State I (60.35 ± 21.71%) was higher than State II (39.65 ± 21.71%). Compared to HC, both TLE‐HS groups showed lower fractional windows in State I and higher fractional windows in State II (all p < .01) (Figure 2c). Additionally, we observed lower frequency of occurrence in State I and higher frequency of occurrence in State II in TLE‐MD (State I: 31.32 ± 18.36%, State II: 68.68 ± 18.36%) compared to TLE‐MI (State I: 45.33 ± 21.75%; State II: 54.67 ± 21.75%, p < .01).
FIGURE 2.
Temporal properties analysis in two discrete functional connectivity states of the hippocampal network. (a) State I is characterized by anterior hippocampal network dominated pattern, which represents positive coupling within/between modules of the medial temporal lobe (MTL) and anterior extra‐MTL. (b) State II is characterized by posterior hippocampal network dominated pattern, which represents positive coupling within posterior extra‐MTL module, as well as positive correlation between posterior extra‐MTL module and each other modules. The percentage of fractional windows (c), mean dwell time (d), and number of transitions (e) in each state is displayed for the healthy controls (HC), temporal lobe epilepsy with memory intact (TLE‐MI) patients, and TLE with memory deficit (TLE‐MD) patients. All of the significance levels were set to p < .05 with multiple comparisons correction. *p < .05, **p < .01, ***p < .001; error bars represent SDs. AMYG, amygdala; ANG, angular gyrus; FUS, anterior fusiform gyrus; ITC, anterior inferior temporal cortex; OFC, lateral orbitofrontal cortex; PCC, posterior cingulate cortex; PHC, parahippocampal cortex; PRC, perirhinal cortex; PREC, precuneus; RSC, retrosplenial cortex
Significant differences in the mean dwell time of each state were observed among groups (State I: F = 10.7984, p < .001; State II: F = 7.6450, p < .001). The post hoc analysis revealed that the mean dwell time of State I was shortest in TLE‐MD and longest in HC (Figure 2d). Accordingly, the mean dwell time in State II was longer in the TLE‐MD group than that in HC (p < .005) as well as TLE‐MI (p < .05). No differences among groups were found in the number of transitions between states (F = 1.2026, p = .2048) (Figure 2e).
3.4. Static and dynamic modular alteration of the hippocampal network
3.4.1. Overall modular properties
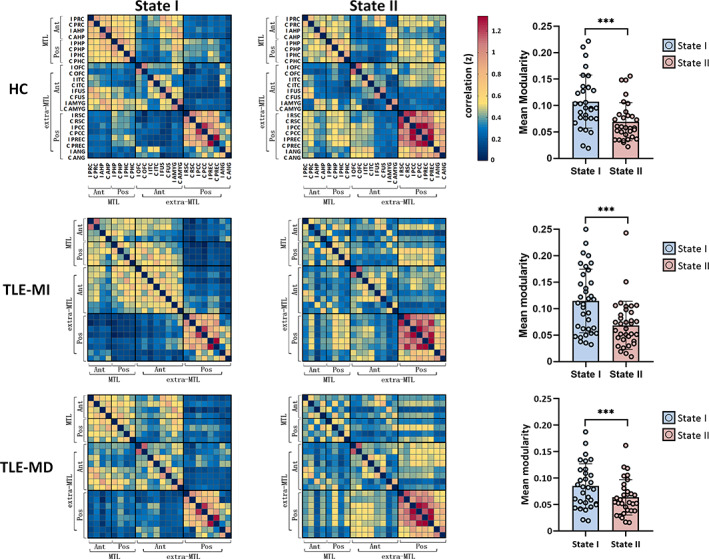
The mean global modularity of State I was higher than that of State II in each group by using paired permutation tests (all p < .005) (Figure 1). A significant difference in global modularity was identified in State I among the three groups (F = 2.8926, p < .05). The mean global modularity measured in State I was lower in TLE‐MD compared to HC (p < .05) as well as TLE‐MI (p < .05). No significant difference in the global modularity was found in either static state or State II among the three groups (static state: F = 1.3263, p = .1498; State II: F = 1.1543, p = .2227).
3.4.2. Intramodular and intermodular connections
In static state analysis, significant group differences in intramodular communication of anterior MTL (F = 11.9545, p < .0001), posterior MTL (F = 11.2306, p < .0001), and intermodular connections between anterior and posterior MTL (F = 6.1897, p < .005) were found. Intramodular communication of anterior and posterior MTL in both patient groups was decreased (all p < .05). Decreased intermodular connections between anterior and posterior MTL were found in TLE‐MD relative to HC (p < .05) (Figure 3).
FIGURE 3.
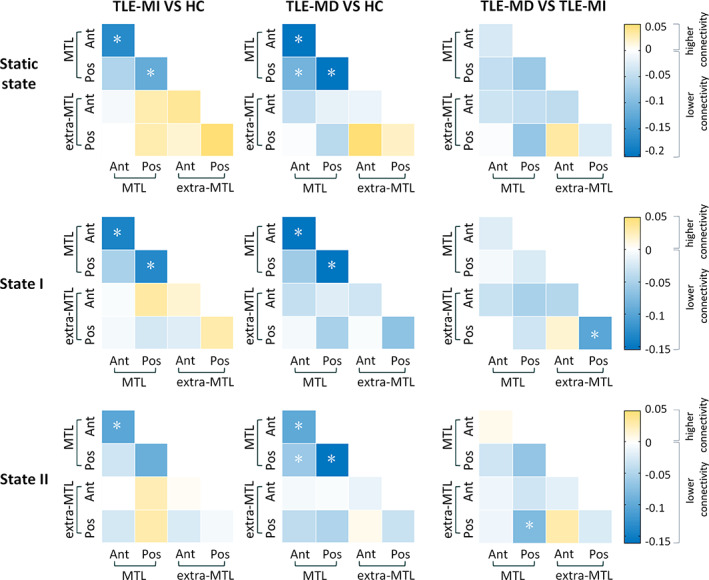
Modular analysis of the hippocampal network in static state and dynamic functional connectivity states. The matrices present between‐group differences in intramodular and intermodular functional connectivity of the hippocampal network for each pair of groups in static state and two discrete functional connectivity states, respectively. The color bar indicates the change in connectivity. All of the significance levels were set to p < .05 with multiple comparisons correction. *p < .05. Ant, anterior; HC, healthy controls; MD, memory deficit; MI, memory intact; MTL, medial temporal lobe; Pos, posterior; TLE, temporal lobe epilepsy
In State I, significant group differences in intramodular communication of anterior MTL (F = 7.2981, p < .005), posterior MTL (F = 6.6068, p < .001), and posterior extra‐MTL (F = 5.6142, p < .005) were revealed. We found common reduced intramodular communication of anterior and posterior MTL in both patient groups (all p < .05). Moreover, TLE‐MD showed lower intramodular connections of posterior extra‐MTL compared with TLE‐MI (p < .05) (Figure 3).
In State II, we found significant group differences in intramodular communication of anterior MTL (F = 6.0935, p < .0001) and posterior MTL (F = 9.5985, p < .0001). Significant group differences were also found in intermodular connectivity between anterior and posterior MTL (F = 3.0795, p < .05) and intermodular connectivity between posterior MTL and posterior extra‐MTL (F = 3.9456, p < .05). The two patient groups exhibited lower intramodular connectivity of anterior MTL than HC (p < .05). The TLE‐MD group had decreased intramodular connectivity within posterior MTL and intermodular connections between anterior and posterior MTL relative to HC (both p < .05). Additionally, the TLE‐MD group exhibited lower intermodular connectivity between posterior MTL and posterior extra‐MTL relative to the TLE‐MI group (p < .05) (Figure 3).
3.4.3. Validation
Dynamic FC analysis identified the hippocampal network with dichotomous FC states: a highly modularized state and a lowly modularized state. These connectivity patterns of identified dynamic FC states are highly similar to the original results and across different window sizes. In addition, group comparisons on modular and temporal features of dynamic FC states keep consistent with different window sizes (see Supporting Information Figures S4–S11 in Appendix S1).
Through identifying the differences in temporal and modular properties using the 2 × 2 factorial ANOVA, we found that there was a main effect of TLE‐MI versus TLE‐MD (fractional windows of State I: F = 7.6959, p < .01; mean dwell time of State I: F = 4.1655, p < .05; mean dwell time of State II: F = 4.8681, p < .05; intramodular connections of posterior extra‐MTL in State I: F = 7.9755, p < .005; intermodular connectivity between posterior MTL and posterior extra‐MTL in State II: F = 8.3179, p < .005), while there were no significant main effects for seizure lateralization or interaction effects (all p > .05).
In the discrimination between TLE‐MI and TLE‐MD, dynamic FC variables of the dwell time in State II and intermodular FC between posterior MTL and posterior extra‐MTL were selected. The sensitivity, specificity, AUC value, and p value were 82.9%, 71.0%, 0.842, and p < .001, respectively.
The ratio of ipsilateral HPV/TIV was significantly lower in TLE‐MD than that in TLE‐MI. In the discrimination between TLE‐MI and TLE‐MD patients, three models were compared to determine whether dynamic FC variables offer unique information for classification of memory impairment. The baseline model including the ratio of ipsilateral HPV/TIV had an AUC significantly above chance (AUC = 0.745, p < .05). The model including dynamic FC variables produced a lager AUC increase (AUC = 0.842) than the baseline model (p < .05). An additional model that included the most important dynamic FC variables as well as the ratio of ipsilateral HPV/TIV performed best (AUC = 0.876) than the other two models (both p < .05) (Figure 4).
FIGURE 4.
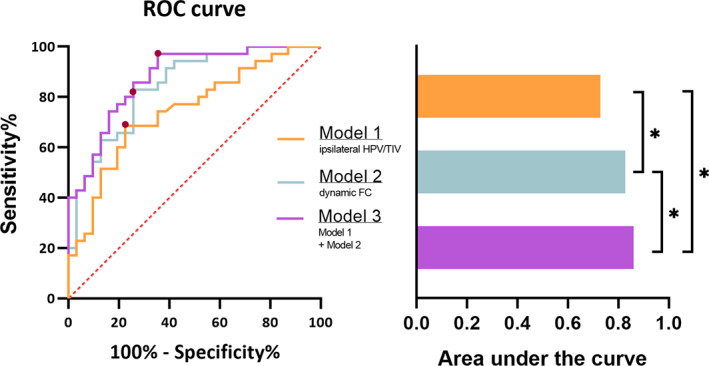
Receiver operating characteristic (ROC) curves and area under the curve (AUC) comparing model performance when discriminating temporal lobe epilepsy with memory deficit (TLE‐MD) from TLE with memory intact (TLE‐MI). (a) The ROC curves associated with three logistic regression models. Models 3 include Model 1 plus Model 2. The red points represent the location of the best discriminative results determined by the Youden index in each ROC. (b) The AUC associated with each ROC curve. Model 2 produced a lager AUC increase (AUC = 0.842) than Model 1 (AUC = 0.745). Model 3 performed best (AUC = 0.876) than the other two models. *p < .05. FC, functional connectivity; HPV, hippocampal volume; TIV, total intracranial volume
4. DISCUSSION
In this study, we investigated the difference of dynamic modular organization of the hippocampal network among healthy subjects and TLE‐HS patients with memory phenotypes in resting‐state fMRI. We had three main findings: (a) there are two different connectivity patterns within the hippocampal network, a high modularized state (State I) with anterior hippocampal network dominated pattern, and a low modularized state (State II) with posterior hippocampal network dominated pattern; (b) TLE was prone to drive less State I but more State II, and the tendency was more obvious in TLE‐MD; (c) TLE‐MD showed more widespread alterations of modular properties compared with TLE‐MI across two states, but the measurement was not sensitive to conventional static FC analysis. Dynamic FC approach has attracted growing attention and may be useful for studying the neural basis of cognition (Deco, Jirsa, & McIntosh, 2011; Lurie et al., 2019). It has been indicted that dynamic FC may reflect language as well as memory functional capacity in TLE (Douw et al., 2015; He et al., 2018). In short, our data demonstrated that dynamic FC analysis of resting‐state fMRI provided insights into fluctuations of the hippocampal network underlying episodic memory impairment in TLE.
Previous research studies demonstrated that dynamic FC analysis could characterize brain segregated and integrated state during rest in healthy subjects and patients with neuropsychiatric disorders (de Pasquale et al., 2012; Kim et al., 2017). Both states of the default mode network were also reported (Du et al., 2016). Here, our findings revealed the hippocampal network with dichotomous FC states: a highly modularized state (State I) and a lowly modularized state (State II). State I and State II may imply this network segregation and integration. Intuitively, State I represented positive coupling within/between modules of anterior extra‐MTL and MTL; State II represent positive coupling within posterior extra‐MTL module, as well as positive correlation between posterior extra‐MTL module and each other modules. It is possible that the discrepancy in anatomical connections influence the intrinsic patterns of functional coupling, which exhibit dense communications in anterior hippocampal network via direct projections and sparse communications in posterior hippocampal network via polysynaptic projections (Poppenk & Moscovitch, 2011; Ranganath & Ritchey, 2012; Ritchey et al., 2015). Briefly, our data that State I had a higher mean global modularity than State II supported the hypothesis that the hippocampal network have segregated and integrated states, and we further indicated that State I was characterized by anterior hippocampal network dominated pattern, and State II was characterized by posterior hippocampal network dominated pattern. However, more evidence is required to confirm the possible mechanism.
Compared to controls, both patient groups have a similar tendency characterized by a reduced occurrence of State I, paralleled by an increased expression of State II. This is in line with the changed temporal properties that patients have a decrease in the dwell time patterns of State I, as well as a corresponding increase of dwell time of State II. Overall, these observations seem to corroborate previous evidence for a selective vulnerability of anterior hippocampal networks in TLE‐HS. It is conceptually understandable that the epileptogenic network mainly occurred in anterior part of hippocampus may represent abnormalities of structure and function in the anterior hippocampal network in TLE‐HS (Barnett, Man, & McAndrews, 2019; Bernasconi et al., 2003; Bernhardt, Bernasconi, Concha, & Bernasconi, 2010; Scanlon et al., 2013). Neuronal loss in the hippocampus may exert a cascading effect that induces proximal and remote limbic deafferentation, which affects functional dynamics (Bernhardt et al., 2019). Thus, our observations underscored that a universal reduction in drive capability of State I due to the damaged integrity of the hippocampus. It is consistent with the result of modular connectivity in both patient groups that a common decrease of intramodular connectivity across anterior to posterior MTL in either State I or State II. This may explain the strengthened temporal dynamics (e.g., frequency and dwell time) of functional integrated State II observed in these patients, implying increased intermodular connections and a widespread integration of memory information across the hippocampal networks to reduce influence of local neural disruption.
The dichotomy of FC states revealed that a progressive imbalance of dynamic networks would aggravate memory impairment in TLE. In other words, the effect of memory preserved through increasing integrated functions is limited if there is an internal functional breakdown in MTL. Importantly, we observed the changes of modular connections across posterior hippocampal network in TLE‐MD, including a reduction of intramodular connections within posterior extra‐MTL during State I and a reduction of intermodular connections between posterior MTL and posterior extra‐MTL during State I. Previous resting fMRI studies in TLE demonstrated that a universal loss of interhemispherical and intra hemispherical FC in posterior hippocampal network reflected worse memory capacity (McCormick et al., 2014; Voets et al., 2014). Posterior hippocampal network is a part of the default mode network, which is engaged in the maintenance of self‐generated cognition and episodic memory. Relative to the anterior hippocampal network, the posterior hippocampal network dominates in maintaining episodic memory (Poppenk & Moscovitch, 2011; Wang et al., 2010). In addition, it could respond to targeted stimulation as a functional unit for recollection improvement (Kim et al., 2018). Interestingly, the dwell time in State II and intermodular FC between posterior MTL and posterior extra‐MTL in State II were identified as the most differentiated variables, which were able to discriminate TLE‐MD from TLE‐MI. Collectively, our finding suggested that memory impairment reflects the breakdown of balance between segregation and integration of the hippocampal network, in which the dynamic changes of posterior hippocampal network play an important role.
In addition, our study showed that static modular analysis failed to provide adequate information in characterizing memory impairment in TLE‐HS. Memory activities require an orchestration of real‐time and continuous brain states, implying a delicate relationship between memory activities and time. Time‐dependent modular organization is more like actual brain dynamics, and is more sensitive in reflecting slight change of memory processing. Moreover, these measurements of temporal dynamics reported were helpful to reveal not only potential neural mechanisms of neuropsychiatric diseases (Damaraju et al., 2014; Jones et al., 2012; Kim et al., 2017; Liu et al., 2016) but also subject‐specific cognition during resting‐state and task‐state (Taghia et al., 2018; Vidaurre, Smith, & Woolrich, 2017). Indeed, our findings demonstrated that dynamic FC analysis have the advantage in providing extra temporal information of different brain states, contributing to better mark episodic memory impairment compared with static features.
We found that the ratio of ipsilateral HPV corrected by HPV/TIV was lower in TLE‐MI group than that in TLE‐MD group. The logistical regression model showed that the ratio of ipsilateral HPV/TIV could differentiate TLE‐MI and TLE‐MD patients. It is well concordance with previous finding that reduction of the HPV ties strongly to memory impairment for TLE‐HS (Dabbs et al., 2009; Hermann, Seidenberg, Lee, Chan, & Rutecki, 2007). This result suggested that the pattern of the further atrophy in the epileptogenic hippocampus could characterize TLE‐MD patients. Further comparison between models showed that the model including dynamic FC variables performed better than the model including the hippocampal volumetric variable, while the model including both dynamic FC metrics and the HPV performed better than the other two models. Therefore, the model comparison demonstrated that fMRI metrics had a greater contribution to memory impairment than structural MRI metrics, and a combination of the two metric types best characterized memory impairment in TLE.
We found TLE‐MD and TLE‐MI patients had no significant difference in seizure lateralization. The TLE‐MD group included patients with either verbal memory impairment (left, 18; right, 10) or nonverbal memory impairment (left, 9; right, 5). About 52% of left TLE‐HS patients and 40% of right TLE‐HS patients were classified as MD. Additionally, there were about 50% left TLE‐HS patients and 33.3% right TLE‐HS patients presenting verbal memory impairment. The trend of distribution was in line with the findings of previous studies, verbal memory impairment was more obviously in left TLE and a considerable proportion of patients in right TLE also had verbal memory impairment (Bell et al., 2011; Giovagnoli & Avanzini, 1999; Helmstaedter & Elger, 2009). Moreover, nonverbal memory impairment is less lateralized than for verbal memory function, thus the structural damage in either side of the mesial temporal lobe is prone to nonverbal memory impairment (Helmstaedter, Pohl, Hufnagel, & Elger, 1991; Hermann, Seidenberg, Schoenfeld, & Davies, 1997; Saling, 2009). Current neuroimaging evidence demonstrated that memory impairment of TLE is associated with the extensive brain network abnormalities, which may be effect by widespread epileptic discharges regardless of seizure lateralization (Dabbs et al., 2009; Rayner et al., 2019; Reyes et al., 2019). We further verified that seizure lateralization has no effects on dynamic modular reorganization in either TLE‐MD or TLE‐MI. This demonstrated that it was feasible to use the properties of dynamic changes in the hippocampal network to mark episodic memory deficits regardless of seizure lateralization.
5. LIMITATIONS
Our study has several limitations. First, the result of the model comparisons indicated that the degree of the hippocampal atrophy and alteration in dynamic FC in the hippocampal network are both independent factors for memory impairment. It is difficult to interpret whether the effect of the alternations of the network FC states on memory is regulated by the HPV. Our study warrants further study to answer this question. Second, a task‐based fMRI could further disclose the characteristics and dynamics of the hippocampal network. An fMRI study during memory processing (encoding, storage and retrieval) will help to further understand the hippocampal–cortical interaction. Third, recruiting MRI‐negative TLE may be helpful to account for whether the local neural loss in the hippocampus ties to temporal alterations of State I. Finally, this study was cross‐sectional, a longitudinal study with enlarged sample size is required to identify the relationship between dynamic FC changes in modular organization of hippocampal network and memory impairment.
6. CONCLUSION
In summary, this study provided a new perspective of understanding alterations in dynamic modular architecture which underlies memory phenotypes in TLE. Dynamic FC analysis of the hippocampal network identified segregated and integrated states that correspond to anterior and posterior hippocampal networks dominated patterns. The sequential intrinsic temporal and functional modular patterns in the hippocampal network were altered in TLE, and associated with memory phenotypes. The trend revealed by dynamic FC is a promising imaging marker for memory impairment in TLE.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Medical Ethic Committee of Second Affiliated Hospital of Zhejiang University School of Medicine.
PATIENT CONSENT STATEMENT
All participants signed the informed consent forms according to the Declaration of Helsinki.
Supporting information
Supplementary Information: Dynamic functional connectivity in modular organization of the hippocampal network marks memory phenotypes in temporal lobe epilepsy
Li, H. , Ding, F. , Chen, C. , Huang, P. , Xu, J. , Chen, Z. , Wang, S. , & Zhang, M. (2022). Dynamic functional connectivity in modular organization of the hippocampal network marks memory phenotypes in temporal lobe epilepsy. Human Brain Mapping, 43(6), 1917–1929. 10.1002/hbm.25763
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 81701670, 81671282; Natural Science Foundation of Zhejiang Province, Grant/Award Number: Q19H180029
Contributor Information
Shuang Wang, Email: wangs77@zju.edu.cn.
Minming Zhang, Email: zhangminming@zju.edu.cn.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request. They are not publicly available due to ethical restrictions.
REFERENCES
- Addis, D. R. , Moscovitch, M. , & McAndrews, M. P. (2007). Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain, 130, 2327–2342. 10.1093/brain/awm166 [DOI] [PubMed] [Google Scholar]
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24, 663–676. 10.1093/cercor/bhs352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, A. J. , Man, V. , & McAndrews, M. P. (2019). Parcellation of the hippocampus using resting functional connectivity in temporal lobe epilepsy. Frontiers in Neurology, 10, 920. 10.3389/fneur.2019.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, B. , Lin, J. J. , Seidenberg, M. , & Hermann, B. (2011). The neurobiology of cognitive disorders in temporal lobe epilepsy. Nature Reviews Neuroscience, 7, 154–164. 10.1038/nrneurol.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi, N. , Bernasconi, A. , Caramanos, Z. , Antel, S. B. , Andermann, F. , & Arnold, D. L. (2003). Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain, 126, 462–469. 10.1093/brain/awg034 [DOI] [PubMed] [Google Scholar]
- Bernhardt, B. C. , Bernasconi, N. , Concha, L. , & Bernasconi, A. (2010). Cortical thickness analysis in temporal lobe epilepsy: Reproducibility and relation to outcome. Neurology, 74, 1776–1784. 10.1212/WNL.0b013e3181e0f80a [DOI] [PubMed] [Google Scholar]
- Bernhardt, B. C. , Fadaie, F. , Liu, M. , Caldairou, B. , Gu, S. , Jefferies, E. , … Bernasconi, N. (2019). Temporal lobe epilepsy: Hippocampal pathology modulates connectome topology and controllability. Neurology, 92, e2209–e2220. 10.1212/WNL.0000000000007447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus, G. , Guedj, E. , Joyeux, F. , Confort‐Gouny, S. , Soulier, E. , Laguitton, V. , … Guye, M. (2009). Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Human Brain Mapping, 30, 1580–1591. 10.1002/hbm.20625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, S. B. , Powell, R. , Yogarajah, M. , Thompson, P. J. , Symms, M. R. , Koepp, M. J. , & Duncan, J. S. (2009). Preoperative amygdala fMRI in temporal lobe epilepsy. Epilepsia, 50, 217–227. 10.1111/j.1528-1167.2008.01739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman, C. A. , Lansink, C. S. , & Pennartz, C. M. (2014). Functions of gamma‐band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. European Journal of Neuroscience, 39, 1982–1999. 10.1111/ejn.12606 [DOI] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. (2010). Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage, 50, 81–98. 10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, R. A. , & Ritchey, M. (2019). Cortico‐hippocampal network connections support the multidimensional quality of episodic memory. eLife, 8, e45591. 10.7554/eLife.45591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs, K. , Jones, J. , Seidenberg, M. , & Hermann, B. (2009). Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy & Behavior, 15, 445–451. 10.1016/j.yebeh.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju, E. , Allen, E. , Belger, A. , Ford, J. , Jacobson McEwen, S. , Mathalon, D. , … Calhoun, V. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical, 5, 298–308. 10.1016/j.nicl.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale, F. , Della Penna, S. , Snyder, A. Z. , Marzetti, L. , Pizzella, V. , Romani, G. L. , & Corbetta, M. (2012). A cortical core for dynamic integration of functional networks in the resting human brain. Neuron, 74, 753–764. 10.1016/j.neuron.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , Jirsa, V. K. , & McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting‐state activity in the brain. Nature Reviews Neuroscience, 12, 43–56. 10.1038/nrn2961 [DOI] [PubMed] [Google Scholar]
- Doucet, G. , Osipowicz, K. , Sharan, A. , Sperling, M. R. , & Tracy, J. I. (2013). Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connectivity, 3, 398–406. 10.1089/brain.2013.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw, L. , Leveroni, C. L. , Tanaka, N. , Emerton, B. C. , Cole, A. J. , Reinsberger, C. , & Stufflebeam, S. M. (2015). Loss of resting‐state posterior cingulate flexibility is associated with memory disturbance in left temporal lobe epilepsy. PLoS One, 10, e0131209. 10.1371/journal.pone.0131209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Pearlson, G. D. , Yu, Q. , He, H. , Lin, D. , Sui, J. , … Calhoun, V. D. (2016). Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophrenia Research, 170, 55–65. 10.1016/j.schres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo, P. , Santana, I. , Teixeira, J. , Cunha, C. , Machado, E. , Sales, F. , … Castelo‐Branco, M. (2008). Adaptive visual memory reorganization in right medial temporal lobe epilepsy. Epilepsia, 49, 1395–1408. 10.1111/j.1528-1167.2008.01629.x [DOI] [PubMed] [Google Scholar]
- Friston, K. (2002). Beyond phrenology: What can neuroimaging tell us about distributed circuitry? Annual Review of Neuroscience, 25, 221–250. 10.1146/annurev.neuro.25.112701.142846 [DOI] [PubMed] [Google Scholar]
- Giovagnoli, A. , & Avanzini, G. (1999). Learning and memory impairment in patients with temporal lobe epilepsy: Relation to the presence, type, and location of brain lesion. Epilepsia, 40, 904–911. 10.1111/j.1528-1157.1999.tb00797.x [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Castillo, J. , Hoy, C. W. , Handwerker, D. A. , Robinson, M. E. , Buchanan, L. C. , Saad, Z. S. , & Bandettini, P. A. (2015). Tracking ongoing cognition in individuals using brief, whole‐brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America, 112, 8762–8767. 10.1073/pnas.1501242112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj, E. , Bettus, G. , Barbeau, E. J. , Liégeois‐Chauvel, C. , Confort‐Gouny, S. , Bartolomei, F. , … Guye, M. (2011). Hyperactivation of parahippocampal region and fusiform gyrus associated with successful encoding in medial temporal lobe epilepsy. Epilepsia, 52, 1100–1109. 10.1111/j.1528-1167.2011.03052.x [DOI] [PubMed] [Google Scholar]
- He, X. , Bassett, D. S. , Chaitanya, G. , Sperling, M. R. , Kozlowski, L. , & Tracy, J. I. (2018). Disrupted dynamic network reconfiguration of the language system in temporal lobe epilepsy. Brain, 141, 1375–1389. 10.1093/brain/awy042 [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C. , & Elger, C. E. (2009). Chronic temporal lobe epilepsy: A neurodevelopmental or progressively dementing disease? Brain, 132, 2822–2830. 10.1093/brain/awp182 [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C. , Pohl, C. , Hufnagel, A. , & Elger, C. E. (1991). Visual learning deficits in nonresected patients with right temporal lobe epilepsy. Cortex, 27, 547–555. 10.1016/s0010-9452(13)80004-4 [DOI] [PubMed] [Google Scholar]
- Hermann, B. , Seidenberg, M. , Lee, E. J. , Chan, F. , & Rutecki, P. (2007). Cognitive phenotypes in temporal lobe epilepsy. Journal of the International Neuropsychological Society, 13, 12–20. 10.1017/S135561770707004X [DOI] [PubMed] [Google Scholar]
- Hermann, B. P. , Seidenberg, M. , Schoenfeld, J. , & Davies, K. (1997). Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Archives of Neurology, 54, 369–376. 10.1001/archneur.1997.00550160019010 [DOI] [PubMed] [Google Scholar]
- Hill, P. F. , King, D. R. , Lega, B. C. , & Rugg, M. D. (2020). Comparison of fMRI correlates of successful episodic memory encoding in temporal lobe epilepsy patients and healthy controls. NeuroImage, 207, 116397. 10.1016/j.neuroimage.2019.116397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , … Chang, C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Gati, J. S. , Everling, S. , & Menon, R. S. (2013). Resting‐state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human Brain Mapping, 34, 2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. T. , Vemuri, P. , Murphy, M. C. , Gunter, J. L. , Senjem, M. L. , Machulda, M. M. , … Jack, C. R., Jr. (2012). Non‐stationarity in the "resting brain's" modular architecture. PLoS One, 7, e39731. 10.1371/journal.pone.0039731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner, E. , Reyes, A. , Macari, A. C. , Chang, Y. H. , Paul, B. M. , Hermann, B. P. , & McDonald, C. R. (2019). Identifying the neural basis of a language‐impaired phenotype of temporal lobe epilepsy. Epilepsia, 60, 1627–1638. 10.1111/epi.16283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Criaud, M. , Cho, S. S. , Díez‐Cirarda, M. , Mihaescu, A. , Coakeley, S. , … Strafella, A. P. (2017). Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain, 140, 2955–2967. 10.1093/brain/awx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Nilakantan, A. , Hermiller, M. , Palumbo, R. , Vanhaerents, S. , & Voss, J. (2018). Selective and coherent activity increases due to stimulation indicate functional distinctions between episodic memory networks. Science . Advances, 4, eaar2768. 10.1126/sciadv.aar2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, N. , & Van De Ville, D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage, 104, 430–436. 10.1016/j.neuroimage.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Li, H. , Ji, C. , Zhu, L. , Huang, P. , Jiang, B. , Xu, X. , … Wang, S. (2017). Reorganization of anterior and posterior hippocampal networks associated with memory performance in mesial temporal lobe epilepsy. Clinical Neurophysiology, 128, 830–838. 10.1016/j.clinph.2017.02.018 [DOI] [PubMed] [Google Scholar]
- Liu, F. , Wang, Y.‐F. , Li, M. , Wang, W. , Li, R. , Zhang, Z. , … Chen, H. (2016). Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic‐clonic seizure. Human Brain Mapping, 38, 957–973. 10.1002/hbm.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, S. (1982). Least squares quantization in PCM. IEEE Transactions on Information Theory, 28, 129–137. 10.1109/TIT.1982.1056489 [DOI] [Google Scholar]
- Lurie, D. , Kessler, D. , Bassett, D. , Betzel, R. , Breakspear, M. , Keilholz, S. , … Calhoun, V. (2019). On the nature of resting fMRI and time‐varying functional connectivity.
- McCormick, C. , Protzner, A. B. , Barnett, A. J. , Cohn, M. , Valiante, T. A. , & McAndrews, M. P. (2014). Linking DMN connectivity to episodic memory capacity: What can we learn from patients with medial temporal lobe damage? NeuroImage: Clinical, 5, 188–196. 10.1016/j.nicl.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, B. (1968). Disorders of memory after brain lesions in man. Preface: Material‐specific and generalized memory loss. Neuropsychologia, 6, 175–179. [Google Scholar]
- Pascual‐Marqui, R. D. , Michel, C. M. , & Lehmann, D. (1995). Segmentation of brain electrical activity into microstates: Model estimation and validation. IEEE Transactions on Biomedical Engineering, 42, 658–665. 10.1109/10.391164 [DOI] [PubMed] [Google Scholar]
- Poppenk, J. , & Moscovitch, M. (2011). A hippocampal marker of recollection memory ability among healthy young adults: Contributions of posterior and anterior segments. Neuron, 72, 931–937. 10.1016/j.neuron.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Powell, H. , Richardson, M. , Symms, M. , Boulby, P. , Thompson, P. , Duncan, J. , & Koepp, M. (2007). Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia, 48, 1512–1525. 10.1111/j.1528-1167.2007.01053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath, C. , & Ritchey, M. (2012). Two cortical systems for memory‐guided behaviour. Nature Reviews Neuroscience, 13, 713–726. 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Rayner, G. , Tailby, C. , Jackson, G. , & Wilson, S. (2019). Looking beyond lesions for causes of neuropsychological impairment in epilepsy. Neurology, 92, e680–e689. 10.1212/WNL.0000000000006905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, A. , Kaestner, E. , Bahrami, N. , Balachandra, A. , Hegde, M. , Paul, B. M. , … McDonald, C. R. (2019). Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology, 92, e1957–e1968. 10.1212/wnl.0000000000007370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey, M. , Libby, L. A. , & Ranganath, C. (2015). Cortico‐hippocampal systems involved in memory and cognition: The PMAT framework. Progress in Brain Research, 219, 45–64. 10.1016/bs.pbr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Roger, E. , Pichat, C. , Torlay, L. , & David, O. (2020). Hubs disruption in mesial temporal lobe epilepsy. A resting‐state fMRI study on a language‐and‐memory network. Human Brain Mapping, 41, 779–796. 10.1002/hbm.24839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling, M. M. (2009). Verbal memory in mesial temporal lobe epilepsy: Beyond material specificity. Brain, 132, 570–582. 10.1093/brain/awp012 [DOI] [PubMed] [Google Scholar]
- Scanlon, C. , Mueller, S. G. , Cheong, I. , Hartig, M. , Weiner, M. W. , & Laxer, K. D. (2013). Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. Journal of Neurology, 260, 2320–2329. 10.1007/s00415-013-6974-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu, M. , Stretton, J. , Winston, G. , Bonelli, S. , Centeno, M. , Vollmar, C. , … Duncan, J. (2013). A functional magnetic resonance imaging study mapping the episodic memory encoding network in temporal lobe epilepsy. Brain, 136, 1868–1888. 10.1093/brain/awt099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. , & Betzel, R. F. (2016). Modular brain networks. Annual Review of Psychology, 67, 613–640. 10.1146/annurev-psych-122414-033634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L. R. , & Zola‐Morgan, S. (1991). The medial temporal lobe memory system. Science, 253, 1380–1386. 10.1126/science.1896849 [DOI] [PubMed] [Google Scholar]
- Taghia, J. , Cai, W. , Ryali, S. , Kochalka, J. , Nicholas, J. , Chen, T. , & Menon, V. (2018). Uncovering hidden brain state dynamics that regulate performance and decision‐making during cognition. Nature Communications, 9, 2505. 10.1038/s41467-018-04723-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. J. , & Corcoran, R. (1992). Everyday memory failures in people with epilepsy. Epilepsia, 33(Suppl 6), S18–S20. [PubMed] [Google Scholar]
- Vannest, J. , Szaflarski, J. P. , Privitera, M. D. , Schefft, B. K. , & Holland, S. K. (2008). Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy & Behavior, 12, 410–418. 10.1016/j.yebeh.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Vidaurre, D. , Smith, S. , & Woolrich, M. (2017). Brain network dynamics are hierarchically organized in time. Proceedings of the National Academy of Sciences of the United States of America, 114, 12827–12832. 10.1073/pnas.1705120114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets, N. L. , Zamboni, G. , Stokes, M. G. , Carpenter, K. , Stacey, R. , & Adcock, J. E. (2014). Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. Journal of Neuroscience, 34, 4920–4928. 10.1523/JNEUROSCI.4281-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Laviolette, P. , O'Keefe, K. , Putcha, D. , Bakkour, A. , Van Dijk, K. R. , … Sperling, R. A. (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage, 51, 910–917. 10.1016/j.neuroimage.2010.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Guan, X. , Li, H. , Xu, X. , & Zhang, M. (2019). Integration and segregation of functional segmented anterior and posterior hippocampal networks in memory performance. Behavioural Brain Research, 364, 256–263. 10.1016/j.bbr.2019.02.019 [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , & Breakspear, M. (2015). Towards a statistical test for functional connectivity dynamics. NeuroImage, 114, 466–470. 10.1016/j.neuroimage.2015.03.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information: Dynamic functional connectivity in modular organization of the hippocampal network marks memory phenotypes in temporal lobe epilepsy
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. They are not publicly available due to ethical restrictions.


