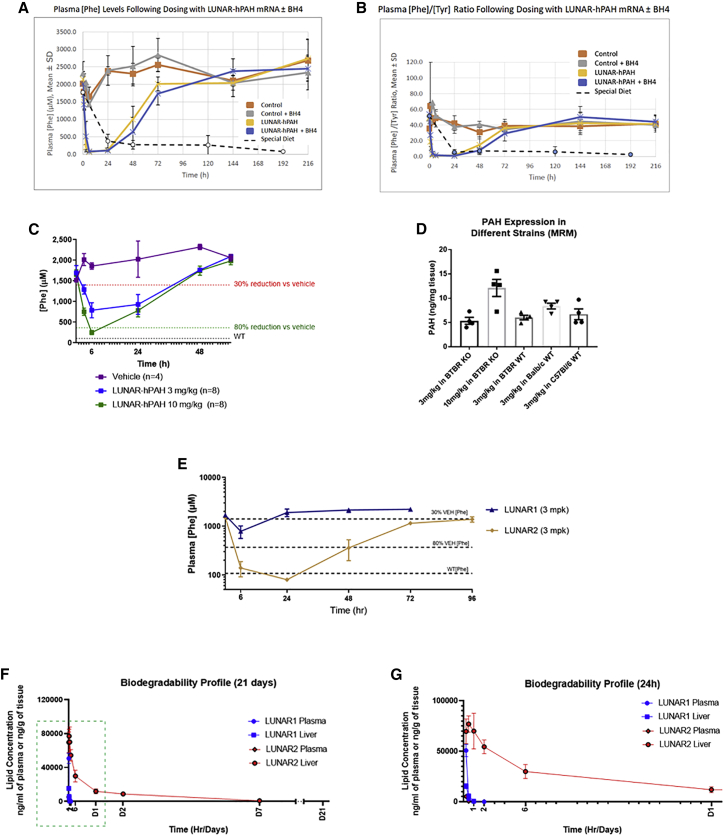
Figure 4.
LUNAR-hPAH mRNA pharmacodynamics in vivo following a single dose
(A and B) Phe plasma levels (A) and Phe:Tyr ratios (B) in Pahenu2 homozygous mice at different time points after a single dose of control (LUNAR buffer) or 10 mg/kg of LUNAR-hPAH mRNA (LUNAR 1) with or without the PAH cofactor BH4. Dashed lines represent values in untreated animals maintained on a special Phe-free diet (n = 3–4 mice in control groups and n = 5–6 in treated groups). (C) Phe plasma levels dose response in Pahenu homozygous mice after a single dose of LUNAR-hPAH mRNA (LUNAR 1) at different concentrations (3 and 10 mg/kg). Gray dashed line indicates average Phe levels in WT mice (n = 8 mice per group). (D) PAH protein quantification by MRM in WT and Pahenu2 homozygous mice from different strains after a single injection of LUNAR-hPAH mRNA (LUNAR 1) (n = 4 mice per group). (E) Plasma Phe levels in Pahenu2 homozygous mice at different time points after a single dose of control (LUNAR buffer) and 3 mg/kg of two different LUNAR formulations (LUNAR1, LUNAR2) encapsulating the same hPAH mRNA (n = 6–8 mice per group). (F and G) Liver and plasma LUNAR lipid concentration quantification at different time points (2 min, 15 min, 30 min, 1 h, 2 h, 6 h, 24 h, 48 h, 7 days, and 21 days post-dose) in WT mice after a single injection of 0.5 mg/kg of LUNAR1 and LUNAR2 mRNAs (n = 4 mice per time point). (G) Detail of the LUNAR lipid concentration during the first 24 h (dashed box in F).