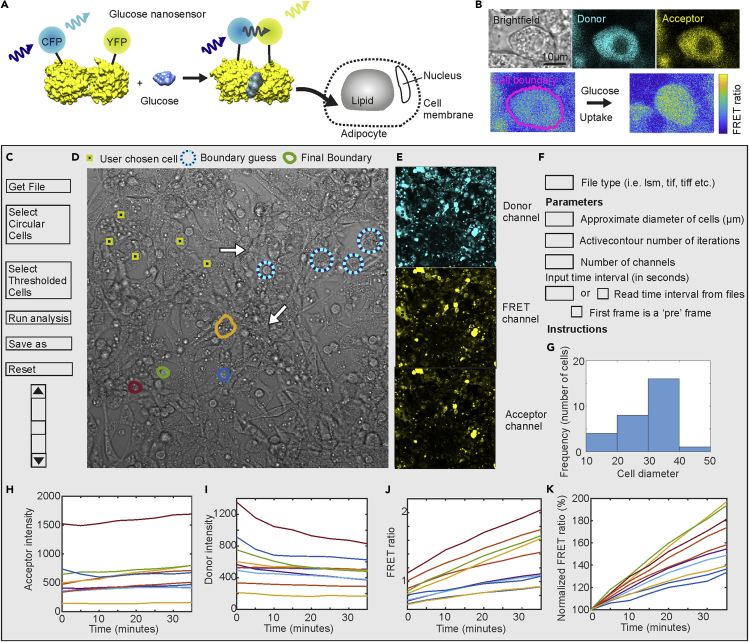
Figure 1.
Glucose nanosensors and FRETzel
(A) Schematic of glucose nanosensor operating in 3T3-L1 adipocytes. Glucose binding induces a conformational change in the glucose-binding protein, bringing the fluorophores into a closer proximity allowing FRET.
(B) Confocal microscopy images of 3T3-L1 adipocytes expressing the sensor. Brightfield generated from the transmitted part of the donor excitation is shown in gray, the donor signal in cyan, and the acceptor in yellow. The pixel-by-pixel FRET ratio is shown below using a parula colormap, with the cell boundary shown in pink. Glucose uptake causes an increase in FRET values. C-K. Mock-up of the software user interface.
(C) Buttons for basic functions.
(D) Transmission confocal image of adipocytes with segmentation steps shown. User first choses cells for analysis (yellow/black squares) and the software places a circle (blue/cyan) of set guess diameter. User then adjusts the circle to approximately match the cell boundary, before the software uses active contouring to define the final cell boundary (colored outlines).
(E) Corresponding fluorescence channels: direct donor excitation (cyan), donor excited acceptor (yellow), and direct acceptor excitation (yellow).
(F) User input parameters.
(G) Histogram distribution of cell diameter (microns).
(H–K). The acceptor (H) and donor (I) intensity, FRET ratio (J) and FRET ratio normalized to the initial value (100%) (K) as a function of time after adding 25mM glucose to glucose starved adipocytes (different colored lines each represent data collected from single cells within the same field contemporaneously).