Fig. 3.
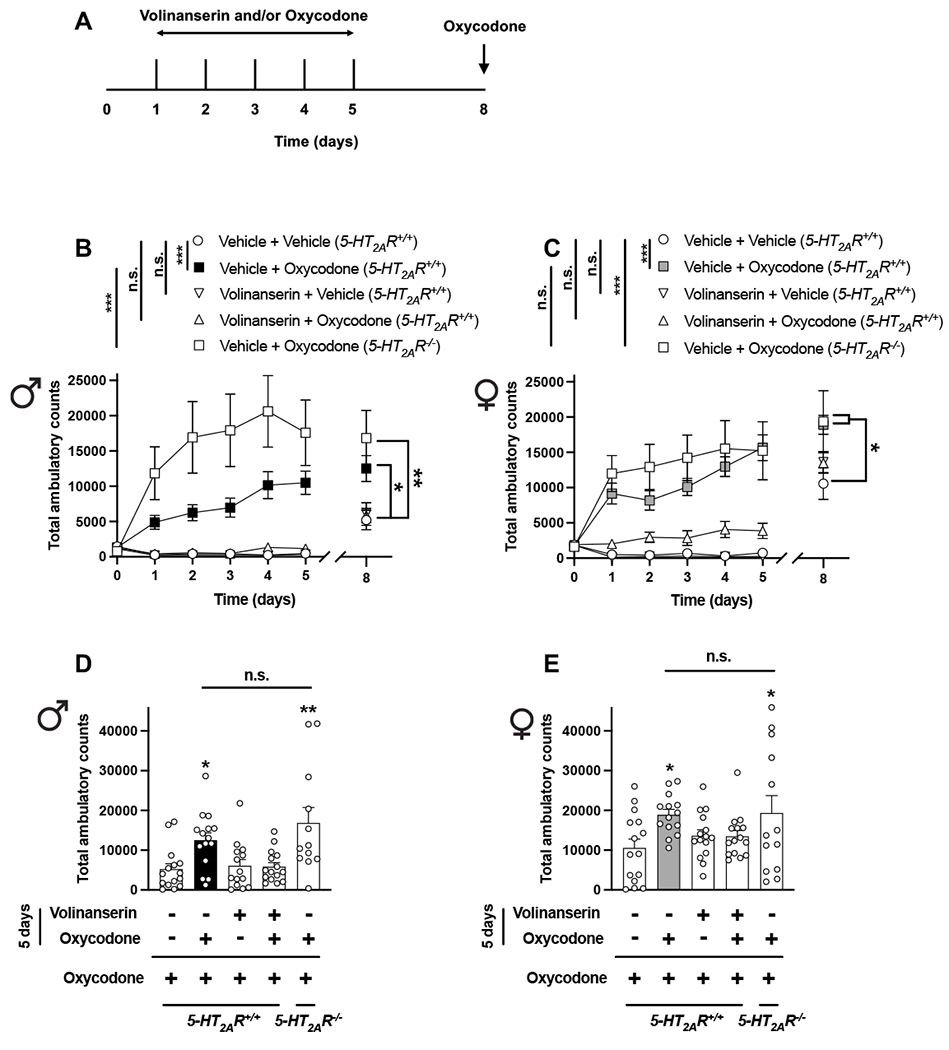
Adjunctive volinanserin prevents oxycodone-induced locomotor sensitization in male and female mice. (A) Timeline of the experimental design. (B-E) Male (B and D) and female (C and E) mice were given oxycodone (8 mg/kg), volinanserin (0.125 mg/kg), volinanserin and oxycodone, or vehicle once a day for 5 days, after which locomotor activity was evaluated. On day 8 (3 days after the last injection), all mice received a single dose of oxycodone (8 mg/kg), and then tested for locomotor activity (n = 12-15 mice per group). Two-way ANOVA (B – days 0-5, Time effect F[5,390] = 5.96, p < 0.001, Volinanserin/Oxycodone/Genotype effect F[4,390] = 64.43, p < 0.001; C – days 0-5, Time effect F[5,402] = 8.32, p < 0.001, Volinanserin/Oxycodone/Genotype effect F[4,402] = 76.31, p < 0.001) or one-way ANOVA (B – day 8 and D, F[4,66] = 6.02, p < 0.001; C – day 8 and E, F[4,67] = 2.59, p < 0.05) followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data are mean ± S.E.M.
