FIGURE 4.
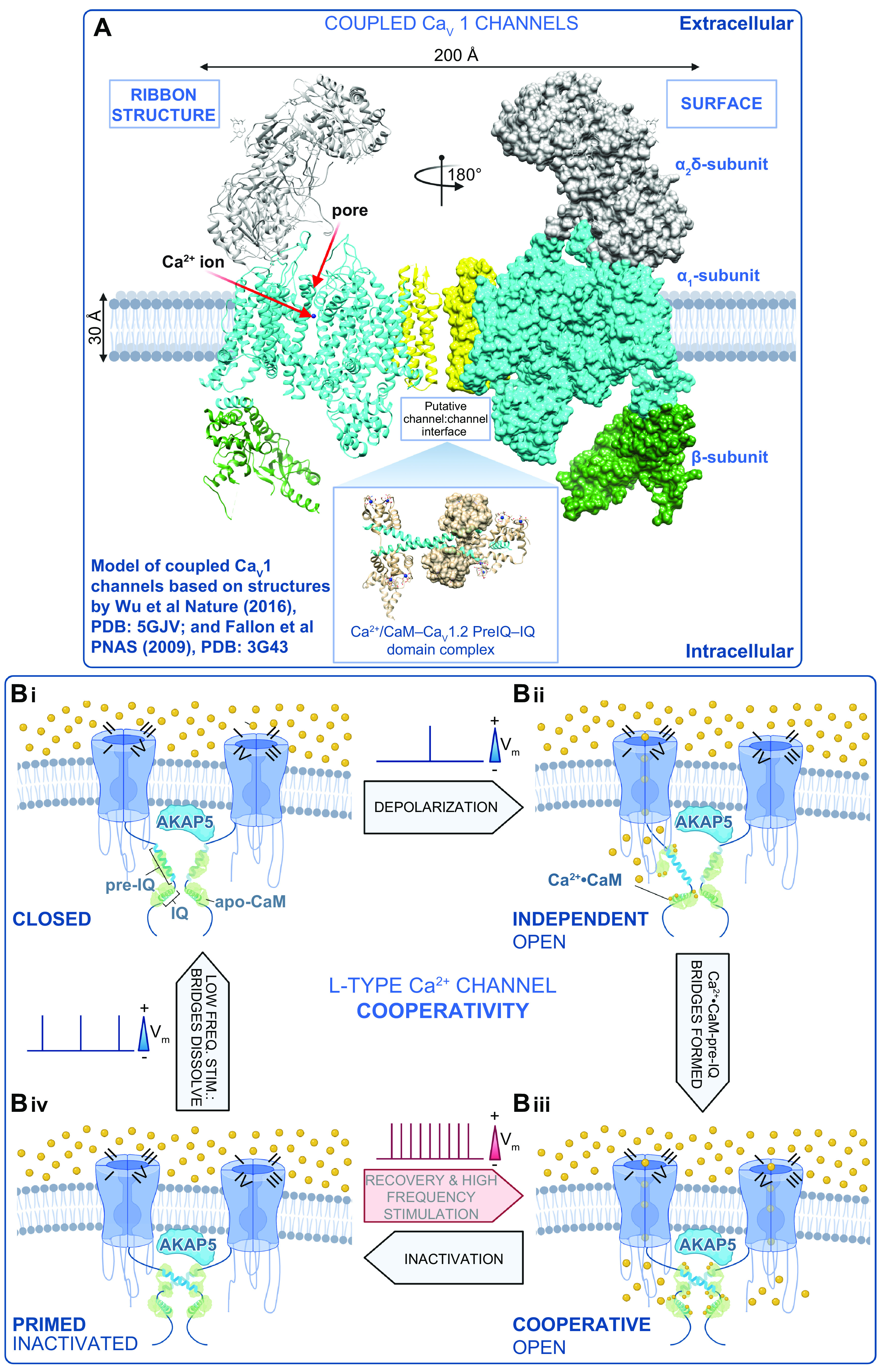
Mechanism of L-type Ca2+ channel cooperative gating. A: model of the interaction between 2 CaV1 channels based on structures by Wu et al. (76) and Fallon et al. (189). B: schematic showing our proposed model of L-type Ca2+ channel cooperative gating based on the analysis of CaV1.2 and CaV1.3 channels (12, 40). Two channels are illustrated for simplicity and are drawn bridged by Ca2+·calmodulin (CaM) in the manner of the 2 published crystal structures of COOH-terminal fragment dimers (189, 190). Although this is speculative at present, we do know that the interactions depend on Ca2+·CaM (12, 42) and intact pre-IQ motifs (12, 42) and occur in AKAP scaffolded microdomains (at least for CaV1.2) (13, 173). For >2 channel multimers to gate coordinately, we postulate that there are at least 2 possibilities: 1) Ca2+·CaM may “daisy-chain” adjacent pre-IQ motifs, utilizing the 2 CaM-binding sites on that motif, or 2) one lobe of Ca2+·CaM may bind to pre-IQ and the other may bind to another of the established CaM binding sites on these channels (for review see Ref. 445). In our scheme, we begin at Bi, where the membrane is at its resting potential and channels are close to one another but not interacting in the closed state. Bii: with depolarization, a subset of the channels stochastically open and Ca2+ flows into the cell, binding to CaM. Biii: Ca2+·CaM facilitates the physical, functional interactions of adjacent channels that depend on binding to the pre-IQ motif. In this functionally cooperative state, the opening of 1 channel is allosterically communicated to the attached channel and they gate coordinately. Biv: CaV channels undergo voltage- and Ca2+-dependent inactivation but remain associated for a time after the Ca2+ signal has decayed, leaving them in a primed state as the cells repolarize. Thus, with high-frequency activation, e.g., in the heart during fight or flight, the channels can immediately transition to the cooperative open state, resulting in an immediate facilitation of Ca2+ influx. During low-frequency stimulation, e.g., during resting heart rate, the physical bridges between adjacent channels in a cluster are dissolved, and the resting confirmation is assumed as the cycle is resumed. Vm, membrane potential. Figure created with Biorender.com.
