Figure 3.
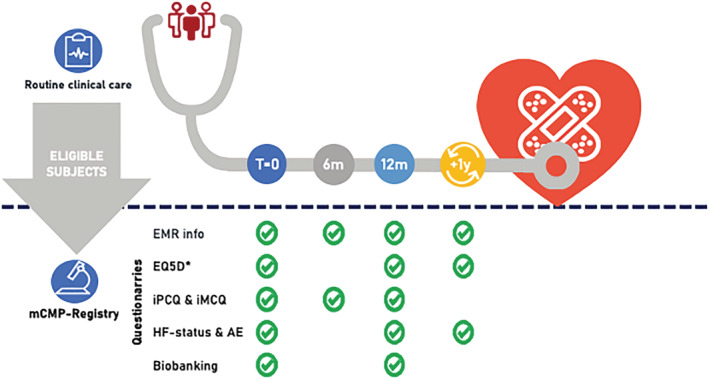
Subjects included in the mCMP‐registry undergo clinical care as usual. Regular clinical visits will be at baseline, 6 and 12 months, and finally yearly unless the treating cardiologist decides otherwise. Upon inclusion in the mCMP‐registry, subjects are asked for additional consent for yearly surveying short questionnaires for a period of 15 years and sequential biobanking. AE, adverse events; EMR, electronic medical records; *EQ‐5D, EuroQol 5D questionnaire (obtained at baseline, and after 1, 3, 5, 10, and 15 years in informed consented subjects); HF, heart failure; iMCQ, iMTA Medical Consumption Questionnaire; iPCQ, iMTA Productivity Cost Questionnaire; m, months; mCMP‐registry, Maastricht Cardiomyopathy registry; T, time.
