Abstract
Aims
Body roundness index (BRI) is an obesity‐related anthropometric index that combines waist circumference and height to better reflect body fat. This study aims to prospectively explore the relationship between BRI and the risk of heart failure (HF) based on a community‐based cohort.
Methods and results
A total of 140 362 individuals without tumour and HF at baseline were included from the Kailuan cohort study. Their demographic information, anthropometric parameters, and biochemical indexes were collected or measured. The participants were followed up until 31 December 2016 or death or diagnosed with HF, whichever came first. Cox proportional hazards model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for incident HF. Restricted cubic spline analysis was applied to further evaluate the possible non‐linear dose–response relationship between BRI and the risk of HF. After a median follow‐up period of 9.84 years, we identified 1990 HF events. The participants were grouped into four groups according to the quartiles of BRI (Q1: ≤2.93, Q2: 2.93–3.59, Q3: 3.59–4.38, and Q4: ≥4.38). After adjustment for potential confounders, compared with the group of participants in the lowest quartile of BRI, the adjusted HRs (95%CI) were 1.03 (95%CI: 0.87–1.22), 1.27 (95%CI: 1.07–1.49), and 1.50 (95%CI: 1.26–1.78) for subjects in the Q2, Q3, and Q4 groups, respectively. With each standard deviation (here is 1.10) of BRI increasing, the risk of HF increased by 18% (HR: 1.18, 95%CI: 1.12–1.24). Subgroup analysis indicated that the association between BRI and HF was more prominent in younger people (HR: 2.94, 95%CI: 1.80–4.80) than older (HR: 1.89, 95%CI: 1.57–2.27) (P for interaction < 0.001). A significant linear dose–response relationship between BRI and HF was also observed (P for non‐linearity = 0.730).
Conclusions
Our study suggests that higher BRI is associated with an increased risk of HF. If these findings can be replicated in other populations, future studies need to examine whether lowering the BRI may lower the risk of incident HF.
Keywords: Body roundness index, BRI, Heart failure, Risk factor, Cohort study
Introduction
Heart failure (HF) is a serious threat to human health in Asia and all over the world. According to statistics, there were about 26 million patients with HF in the world, and the number is expected to increase because of the ageing population trend. 1 , 2 , 3 , 4 There are data to suggest similar trends depicting high prevalence of HF in many Asian countries. 5 , 6 , 7 Over the past few decades, much research has been devoted to identifying the modifiable risk factors for HF. However, there was significantly less research on HF in Asia than in the West, even though Asian patients are younger and have more clinical symptoms. 8 Therefore, more epidemiological studies on the modifiable risk factors of HF in some Asian districts are needed, which may be helpful for the better medical management and policy‐making of HF in Asia.
It is well known that obesity is a major modifiable risk factor for cardiovascular diseases (CVD) and strongly associated with the occurrence of HF. 9 , 10 Compared with the general obesity represented by body mass index (BMI), abdominal obesity has been proposed to be a much stronger risk factor for CVD. 11 , 12 Because BMI can neither reflect the visceral fat nor distinguish between muscle and adipose tissue, BMI may not be an optimal marker of measuring human adiposity. 13 , 14 Asian people, in particular, have more visceral fat at similar BMI levels and are more prone to abdominal obesity than other ethnic groups. 15 Therefore, defining obesity by the standard measure of BMI tends to underestimate the risk of CVD and HF in Asians.
Body roundness index (BRI) is an obesity‐related anthropometric index proposed by Thomas et al. in 2013, which combines waist circumference (WC) and height to describe a person's body shape and better reflects the proportion of body fat and visceral fat than the traditional indexes such as BMI, WC, and hip circumference. 16 Although studies have shown that BRI was a risk factor for various diseases, such as coronary artery disease, carotid atherosclerosis, and diabetes mellitus, 17 , 18 , 19 there is currently no longitudinal cohort study to investigate the association between BRI and HF. Therefore, this study aims to investigate whether BRI is a significant risk factor for HF and to further explore the dose–response relationship between BRI and HF using a large population‐sized Chinese community‐based cohort. Revealing the relationship between BRI and HF may help to develop more comprehensive prevention strategies in preventing the occurrence of HF.
Methods
Study design and population
We obtained the data from a prospective cohort study in the Kailuan community of Tangshan City, China. The study design has been covered in detail in other articles, and this study has been approved by the Ethics Committee of the Kailuan General Hospital, and the investigation conforms with the principles outlined in the Declaration of Helsinki. 20 , 21 , 22 In brief, the Kailuan community is a fully equipped community, with 11 hospitals responsible for community health care. The participants of this study were selected from 159 018 people who agreed and completed the first survey in any of the four phases: 2006/2007, 2008/2009, 2010/2011, and 2012/2013. These participants received physical examination every 2 years in the hospital where questionnaire assessment, clinical examination, and laboratory examination were performed. Inclusion criteria of the present study were set as follows: (i) no history of tumour or HF, (ii) 20–80 years old, and (iii) complete BRI data records. Finally, 140 362 people were included in the present study. The flowchart of inclusion of participants was shown in Figure 1 .
Figure 1.
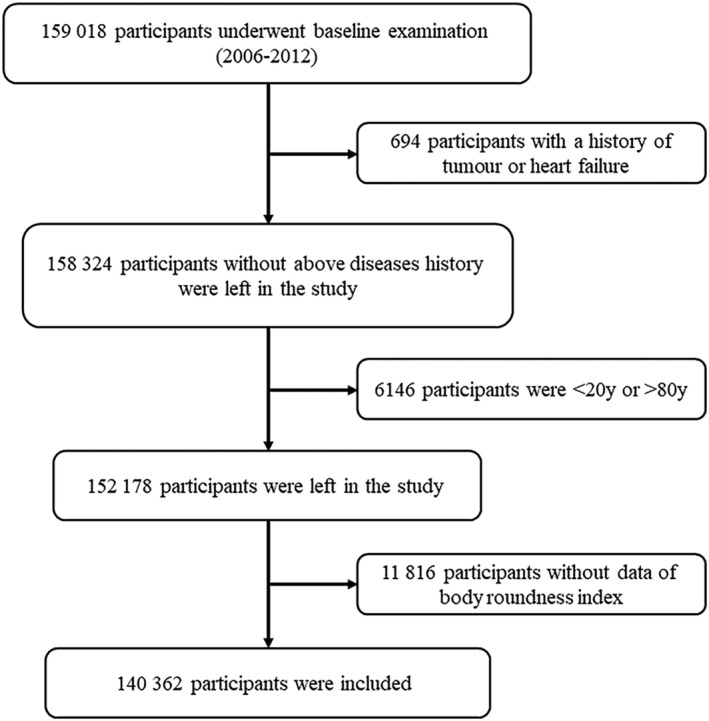
Flowchart of inclusion of participants.
Data collection and definitions
Demographic information (e.g. age, gender, and educational attainment), lifestyle (e.g. smoking, alcohol consumption, and physical activity), and disease history (e.g. HF and tumour) were obtained through questionnaires. Smoking was defined as averaging at least one cigarette a day for more than a year. Drinking was defined as averaging at least 100 mL a day for more than a year. Smoking and drinking status were both divided into three types: current, past, and never. Current means participants were smoking/drinking when the information was collected, past means participants had quitted smoking/drinking when the information was collected, and never means participants had never smoked/drunk before the information was collected. Physical activity was defined as exercising ≥30 min each time for more than three times per week. The participants received physical examinations by trained field workers (i.e. nurses and doctors) to obtain their anthropomorphic parameters, including height, weight, WC, blood pressure (BP), and BMI. BP included systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure. After fasting for 8–12 h, fasting blood samples were collected to measure fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and high‐sensitivity C‐reactive protein (hs‐CRP) levels. Diabetes was defined as if any of the following conditions was true: (i) FBG ≥ 7.0 mmol/L; (ii) use of hypoglycaemic drugs; (iii) self‐reported diabetes history. Hypertension was defined as if any of the following conditions was true: (i) BP ≥ 140/90 mmHg; (ii) use of antihypertensive drugs; (iii) self‐reported hypertension history. Hyperlipidaemia was defined as if any of the following conditions was true: (i) TC ≥ 5.17 mmol/L, TG ≥ 1.69 mmol/L, low‐density lipoprotein cholesterol ≥ 3.62 mmol/L, or high‐density lipoprotein cholesterol ≤ 1.04 mmol/L; (ii) use of lipid‐lowering drugs; and (iii) self‐reported hyperlipidaemia history. 23
Calculation of body roundness index
The calculation formula of BRI is as follows: , WC and height are both in metres. 16
Follow‐up and assessment of heart failure
Subjects enrolled in physical examination of 2006, 2008, 2010, or 2012 were followed up until 31 December 2016 or death or diagnosed with HF, whichever came first. The diagnosis of HF was based on the criteria established by the European Society of Cardiology. 24 The specific type of HF in the present study was chronic systolic HF and the diagnostic criteria included clinical manifestations, laboratory tests, and imageology. General cardiologists reviewed the medical records of patients and proved the diagnosis of HF according to the following criteria: (i) the clinical features of HF, such as difficulty in breathing, weakness, and fluid retention (e.g. ascites, pleural effusion, pedal oedema, and increased jugular venous pressure), diagnosed with the New York Heart Association cardiac function class ≥ II or Killip cardiac function class ≥ II; (ii) Doppler echocardiography showed the left ventricular ejection fraction ≤ 50%; (iii) increased level of N‐terminal pro‐B‐type natriuretic peptide. The diagnosis of HF was confirmed by the presence of (i) and any of (ii) and (iii).
Statistical analysis
The physical examination data were input into the computer terminal of each hospital by trained nurses and uploaded to Oracle 10.2 database in Kailuan General Hospital. Continuous variables were expressed as the mean ± standard deviation (SD) or the medians with interquartile ranges depending on their distribution, and the difference between groups was compared by one‐way analysis of variance or Kruskal–Wallis test. The categorical variables were expressed as frequencies and percentages, and the χ 2 test was applied for difference comparison. Kaplan–Meier curve and log‐rank test were used to reflect the cumulative incidence of HF across the quartiles of BRI. According to Schoenfeld residuals and log–log inspection, there was no significant departure from proportionality in hazards over time. Cox proportional hazards model was used to assess the relationship between BRI (continuous variable or grouped according to quartiles) and the risk of incident HF events, with adjustment for age, sex, educational attainment, salt intake, physical activity, smoking, drinking, diabetes, hypertension, hyperlipidaemia, hs‐CRP, and BMI. To further examine potential modification effects by age and sex, subgroup analysis was performed by age (<50, ≥50 years) and sex (women, men). And their interaction effect was estimated by the Wald test. In addition, restricted cubic spline analysis was applied to further evaluate the possible non‐linear dose–response relationship between BRI and the risk of HF, with three knots placed at 10th, 50th, and 90th percentiles. The median BRI (3.59) was set as the reference knot. To avoid possible reverse causality, the participants with HF occurring in the first 2 years of follow‐up were excluded for sensitivity analysis. We performed all statistical analyses using R Version 4.0.2 statistical software (R Foundation for Statistical Computing, https://www.r‐project.org/). A two‐side P < 0.05 was regarded as significantly different.
Results
Baseline characteristics
A total of 140 362 people aged 20 to 80 years were included in our study, including 114 023 male individuals and 26 339 female individuals. The general baseline characteristics according to the quartiles of BRI are displayed in Table 1 . Individuals with higher BRI were more likely to be older, have more exercise and higher levels of BP, FBG, TC, TG, and hs‐CRP, and have a higher percentage of being diagnosed with diabetes, hypertension, and hyperlipidaemia (all P < 0.001).
Table 1.
Baseline characteristics of the participants according to the quartiles of body roundness index
| Q1 (≤2.93) | Q2 (2.93–3.59) | Q3 (3.59–4.38) | Q4 (≥4.38) | P | |
|---|---|---|---|---|---|
| N | 34 822 | 35 246 | 35 111 | 35 183 | |
| Age (years) | 43.6 ± 13.7 | 48.4 ± 12.5 | 50.7 ± 12.2 | 53.8 ± 12.2 | <0.001 |
| Sex, male | 26 465 (76.0) | 29 790 (84.5) | 29 433 (83.8) | 28 335 (80.5) | <0.001 |
| Education | <0.001 | ||||
| <High school | 2113 (6.1) | 2707 (7.7) | 3417 (9.7) | 4493 (12.8) | |
| High school | 20 142 (57.8) | 23 858 (67.7) | 23 782 (67.7) | 24 099 (68.5) | |
| >High school | 12 567 (36.1) | 8681 (24.6) | 7912 (22.5) | 6591 (18.7) | |
| Physical activity, often | 4607 (13.2) | 5040 (14.3) | 5549 (15.8) | 5858 (16.7) | <0.001 |
| Smoking | <0.001 | ||||
| Never | 20 880 (60.0) | 20 305 (57.6) | 20 099 (57.2) | 21 045 (59.8) | |
| Past | 1251 (3.6) | 1584 (4.5) | 1944 (5.5) | 2429 (6.9) | |
| Current | 12 691 (36.4) | 13 357 (37.9) | 13 068 (37.2) | 11 709 (33.3) | |
| Drinking | <0.001 | ||||
| Never | 20 936 (60.1) | 20 146 (57.2) | 19 838 (56.5) | 21 351 (60.7) | |
| Past | 768 (2.2) | 982 (2.8) | 1237 (3.5) | 1462 (4.2) | |
| Current | 13 118 (37.7) | 14 118 (40.1) | 14 036 (40.0) | 12 370 (35.2) | |
| Salt intake | <0.001 | ||||
| <6 g/day | 4038 (11.6) | 3810 (10.8) | 3870 (11.0) | 3397 (9.7) | |
| 6–10 g/day | 27 274 (78.3) | 27 817 (78.9) | 27 232 (77.6) | 27 501 (78.2) | |
| >10 g/day | 3510 (10.1) | 3619 (10.3) | 4009 (11.4) | 4285 (12.2) | |
| BMI (kg/m2) | 22.1 ± 2.6 | 24.2 ± 2.5 | 25.7 ± 2.6 | 27.8 ± 3.3 | <0.001 |
| WC (cm) | 76 (72–80) | 84 (81–86) | 90 (87–92) | 98 (94–102) | <0.001 |
| FBG (mmol/L) | 5.0 (4.6–5.5) | 5.1 (4.7–5.7) | 5.2 (4.7–5.8) | 5.3 (4.8–6.0) | <0.001 |
| SBP (mmHg) | 120.0 (110.0–130.0) | 125.0 (115.0–140.0) | 130.0 (120.0–141.3) | 132.0 (120.0–150.0) | <0.001 |
| DBP (mmHg) | 80.0 (70.0–83.7) | 80.0 (78.0–90.0) | 81.3 (80.0–90.0) | 84.7 (80.0–92.0) | <0.001 |
| MAP (mmHg) | 93.3 (83.8–100.0) | 96.2 (90.0–106.4) | 97.9 (92.6–108.0) | 101.3 (93.3–112.1) | <0.001 |
| TC (mmol/L) | 4.7 (4.1–5.3) | 4.9 (4.2–5.5) | 5.0 (4.3–5.6) | 5.0 (4.4–5.7) | <0.001 |
| TG (mmol/L) | 1.0 (0.7–1.4) | 1.2 (0.9–1.8) | 1.4 (1.0–2.1) | 1.6 (1.1–2.4) | <0.001 |
| HDL‐C (mmol/L) | 1.5 (1.3–1.7) | 1.5 (1.2–1.7) | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | <0.001 |
| LDL‐C (mmol/L) | 2.4 (1.9–2.8) | 2.4 (2.0–2.9) | 2.4 (1.9–3.0) | 2.4 (1.8–2.9) | <0.001 |
| hs‐CRP | 1.7 ± 4.9 | 2.1 ± 5.6 | 2.5 ± 6.1 | 3.2 ± 7.4 | <0.001 |
| Diabetes | 1400 (4.0) | 2548 (7.2) | 3501 (10.0) | 4999 (14.2) | <0.001 |
| Hypertension | 8435 (24.2) | 13 529 (38.4) | 15 996 (45.6) | 19 487 (55.4) | <0.001 |
| Hyperlipidaemia | 7327 (21.0) | 10 806 (30.7) | 13 552 (38.6) | 16 169 (46.0) | <0.001 |
N, number; BMI, body mass index; WC, waist circumstance; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; TC, total cholesterol; TG, triglyceride; LDL‐C, low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein.
Continuous variables are presented as mean ± standard deviation (normally distributed) or median with 25–75th percentile (not normally distributed); categorical variables are expressed as percentage.
Incidence of heart failure according to the quartiles of body roundness index
After a median follow‐up period of 9.84 years, we identified 1990 incident HF cases. As shown in Table 2 , the number of HF events in Q1, Q2, Q3, and Q4 groups was 224, 338, 532, and 896, respectively. And the corresponding incidence rates of HF were 0.77, 1.12, 1.72, and 2.84 per 1000 person‐years, respectively. Further Kaplan–Meier curve showed that participants in the highest quartile of BRI were more likely to have an increased incidence of HF, compared with those in the lowest quartile of BRI (P < 0.0001, Figure 2 ).
Table 2.
Hazard ratios (95% confidence intervals) for incident heart failure associated with quartiles of body roundness index
| BRI | No. HF | Crude incidence (per 1000 person‐year) | Model 0 | Model 1 | Model 2 |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Q1 (≤2.93) | 224 | 0.77 | Reference | Reference | Reference |
| Q2 (2.93–3.59) | 338 | 1.12 | 1.15 (0.97–1.36) | 1.06 (0.89–1.25) | 1.03 (0.87–1.22) |
| Q3 (3.59–4.38) | 532 | 1.72 | 1.54 (1.31–1.80) | 1.32 (1.13–1.55) | 1.27 (1.07–1.49) |
| Q4 (≥4.38) | 896 | 2.84 | 2.07 (1.79–2.40) | 1.62 (1.39–1.89) | 1.50 (1.26–1.78) |
| P for trend | <0.001 | <0.001 | <0.001 |
BRI, body roundness index; CI, confidence interval; HF, heart failure; HR, hazard ratio; SD, standard deviation.
Model 0: adjusted for age and sex. Model 1: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, and hs‐CRP. Model 2: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, hs‐CRP, and BMI.
Figure 2.
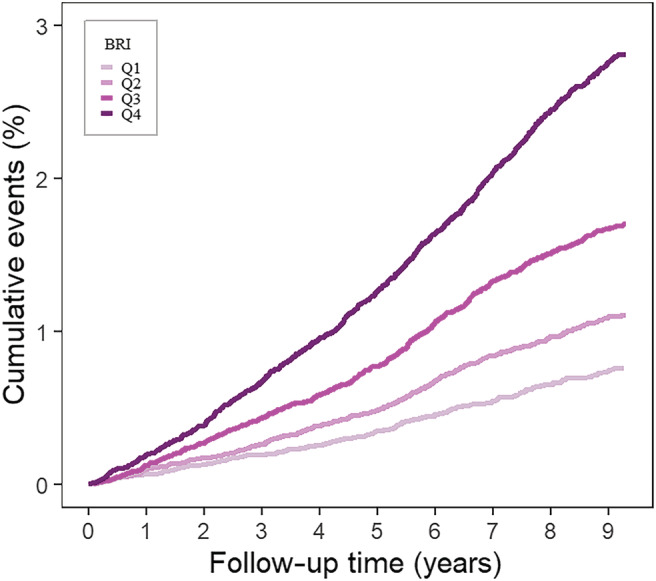
Kaplan–Meier curve of incidence of heart failure according to the quartiles of body roundness index in 140 362 Chinese people followed for a median of 9.84 years. Note: Log‐rank test showed P < 0.0001.
Relationship between body roundness index and the risk of heart failure
Tables 2 and 3 present the results of multivariable Cox regression analysis regarding the relationship between BRI and the risk of HF. After adjusting for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, hs‐CRP, and BMI, with each standard deviation (SD, 1.10) of BRI increasing, the risk of incident HF elevated by 18% (HR: 1.18, 95%CI: 1.12–1.24). In categorical analysis, compared with the Q1 group, the HRs of new‐onset HF in Q2, Q3, and Q4 groups were 1.03 (95%CI: 0.87–1.22), 1.27 (95%CI: 1.07–1.49), and 1.50 (95%CI: 1.26–1.78), respectively (P value for trend < 0.001). In the results of subgroup analysis (Figure 3 ), the adjusted HR (95% CI) of incident HF in participants with lower age (<50 years) was 2.94 (95%CI: 1.80–4.80), while the adjusted HR (95% CI) in participants with higher age (≥50 years) was 1.89 (95%CI: 1.57–2.27). The interaction effect between BRI and age was significant (P for interaction < 0.001). We did not observe a statistically significant interaction effect between BRI and sex (P for interaction = 0.667).
Table 3.
Hazard ratios (95% confidence intervals) for incident heart failure of sensitivity analysis
| BRI | Model 0 | Model 1 | Model 2 |
|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Q1 | Reference | Reference | Reference |
| Q2 | 1.17 (0.97–1.42) | 1.08 (0.89–1.30) | 1.05 (0.87–1.28) |
| Q3 | 1.55 (1.30–1.85) | 1.35 (1.13–1.62) | 1.30 (1.08–1.57) |
| Q4 | 2.15 (1.82–2.54) | 1.73 (1.46–2.05) | 1.62 (1.33–1.97) |
| P for trend | <0.001 | <0.001 | <0.001 |
BRI, body roundness index; CI, confidence interval; HR, hazard ratio.
Model 0: adjusted for age and sex. Model 1: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, and hs‐CRP. Model 2: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, hs‐CRP, and BMI.
Figure 3.
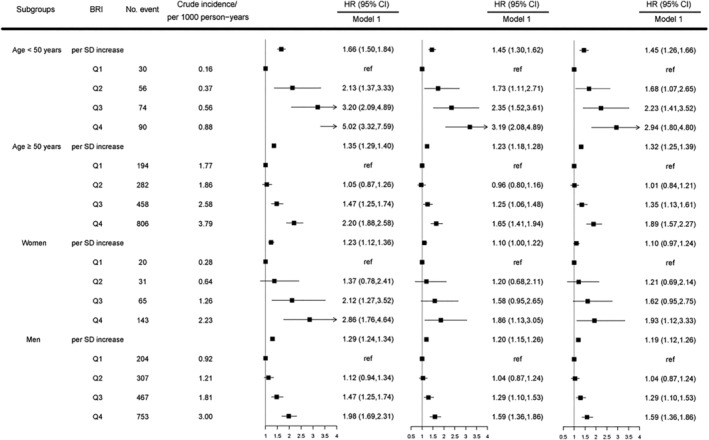
Hazard ratios (95% confidence intervals) for incident heart failure associated with quartiles of body roundness index in the subgroups of age and sex, respectively. Note: The P values for interactions were <0.001 and 0.667 for age and sex, respectively. HR, hazard ratio; CI, confidence interval; BRI, body roundness index; HF, heart failure. Model 0: adjusted for age and sex. Model 1: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, and hs‐CRP. Model 2: adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, hs‐CRP, and BMI.
The dose–response relationship between body roundness index and heart failure
Figure 4 shows the dose–response relationship between BRI and HF risk. The results showed a consistent, positive dose–response association between BRI and the risk of HF. Overall, there was not any departure from linearity in the study participants (P for non‐linearity = 0.730).
Figure 4.

The dose–response relationship between BRI and heart failure. Point estimates (solid line) and 95% confidence intervals (dashed lines) were estimated by restricted cubic splines analysis with knots placed at the 10th, 50th, and 90th percentile (median as the reference). Models were adjusted for age, sex, educational attainment, salt intake, physical activity, smoking, alcohol drinking, diabetes, hyperlipidaemia, hypertension, hs‐CRP, and BMI.
Sensitivity analysis
To avoid the effect of possible reverse causality, we further excluded participants who had HF in the first two follow‐up years in the sensitivity analysis. Similarly, the risk of HF was observed to increase with BRI level (Table 3 ).
Discussion
As far as we know, this is the first prospective cohort study to investigate the relationship between BRI and the risk of HF. During a median follow‐up period of 9.84 years, we observed that the elevated BRI was significantly associated with an increased risk of incident HF. Interestingly, this association was more pronounced in younger adults (<50 years). In addition, the dose–response curve indicated a linear positive correlation between BRI and HF.
Abdominal obesity is considered to be a strong risk factor for HF. 25 Compared with the assessment of visceral fat by imaging, WC measurement is often used as a simple and easy way to evaluate abdominal obesity. But the main drawback of WC is that it does not consider the influence of height, which may lead to underestimation or overestimation of abdominal obesity in short or tall people. 26 , 27 Therefore, researchers have proposed a new body measurement index, namely BRI. This index was calculated by WC relative to height and had better performance than WC in predicting the percentage of body fat and visceral adipose tissue. 16
Our study found that high BRI was still associated with an increased risk of HF after adjusting for BMI. This result supports the existing conclusion that abdominal obesity is an important risk factor for HF and may be independent of general obesity. A Swedish cohort study (N = 80 360) showed that regardless of the value of BMI, WC could predict the risk of HF. 28 A similar finding was reported in a cohort (N = 59 178) where the risk for HF was linked to WC and waist‐hip ratio. 29 Another study (N = 3075) aiming at older people aged 70 to 79 indicated that the abdominal obesity represented by WC was a stronger risk factor for chronic HF than BMI or fat mass. 30 However, several limitations (e.g. relatively small sample size, self‐reported measurement method, and relatively short follow‐up period) might be included in the above‐mentioned studies. Additionally, given the better performance of BRI than WC in predicting the percentage of body fat and visceral adipose tissue, and no prior study has delineated the role of BRI in the onset of HF, this study may provide significant new evidence on the effect of BRI on HF. In the present study, after adjusting for all potential covariates, we found that a one‐SD increment in BRI was associated with an 18% increased risk of HF, implying that excess abdominal adipose tissue is likely to be responsible for the onset of HF. Therefore, weight control, especially for the abdominal adipose tissue, might be helpful for the prevention of HF. According to the Heart Failure practice guideline formulated by the Heart Failure Society of America that advising BMI < 30 as one of the prevention strategies for HF, 31 our results may help to supplement this recommendation. Due to the limited research and the nature of observational study, it needs more studies including randomized controlled trials to confirm the present findings.
Subgroup analysis showed that the association between BRI and the risk of HF was more prominent in younger adults than older adults, which might emphasize the importance of controlling BRI at a young age. A prospective cohort study showed that obesity in middle‐age increases their risk of developing HF later in life while obesity later in life showed insignificant results, which was in accordance with the present findings. 32 The role that age plays in the relationship between BRI and HF is not well understood. Some studies have shown that the duration of obesity can be positively correlated with the risk of most adverse outcomes including CVD. 33 , 34 , 35 In addition, a recent study found that obesity in early life was associated with significant changes in myocardial geometry and function, indicating early onset of potentially unfavourable alterations in the myocardium. 36 Therefore, we speculate that the age of onset of abdominal obesity may play a critical role in the development of HF, but more observational and experimental studies are needed to confirm the effect of age on the relationship between BRI and HF and to figure out the specific mechanisms.
lthough the underlying mechanism on the association of BRI with the risk of HF has not been clarified yet, there are several hypotheses as follows: First, obesity is associated with many CVD, such as hypertension, dyslipidaemia, and diabetes mellitus, and all of these are risk factors for developing myocardial infarction, some of which might lead to HF in turn. 37 , 38 , 39 , 40 , 41 , 42 Second, obesity may lead to elevated production of proinflammatory cytokines, increased oxidative stress, excess haemodynamic load, and activation of neurohormones, all of which might lead to left ventricular remodelling. 43 , 44 , 45 , 46 Third, obesity may directly damage the heart muscle, causing structural and functional damage of the heart. 47
Our study is the first study to explore the relationship between BRI and HF and is based on a large prospective cohort study involving 140 362 individuals followed up for nearly 10 years. However, there are also some limitations in this study. First, we do not have detailed records of the causes of HF, so we cannot determine which cardiovascular disease progressing to HF is the most affected by elevated BRI. Second, we did not statistically compare the predictive power of BRI, BMI, and WC for new‐onset HF, so we cannot make statistical judgements on the merits of these three indexes. Third, our study focused on chronic systolic HF, the results may be not applicable for other conditions of HF. Fourth, there may be other HF risk factors that we have not adjusted, such as the history of amphetamine drug use. Fifth, because the community belongs to the Kailuan Coal Mining Company, there were more men than women in the cohort. However, the ratio of male individuals to female individuals in the included population was similar to that in the original cohort, and we have also performed a gender subgroup analysis to minimize the impact of gender imbalance on the results. Finally, the participants included in our study were from a community‐based cohort and almost all of them were Chinese Han population, which may limit the extrapolation of the findings.
In conclusion, this study demonstrated that elevated BRI was associated with an increased risk of incident HF, and the association was more evident in young people. More related studies on this issue are needed to confirm our findings in the future.
Conflict of interest
None declared.
Acknowledgements
We appreciate all participants included in this study and the staff of the whole survey teams.
Wang, J. , Wu, M. , Wu, S. , and Tian, Y. (2022) Relationship between body roundness index and the risk of heart failure in Chinese adults: the Kailuan cohort study. ESC Heart Failure, 9: 1328–1337. 10.1002/ehf2.13820.
Jianing Wang and Mingyang Wu contributed equally to this work.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Yaohua Tian, Email: yaohua_tian@hust.edu.cn.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 3. Rajadurai J, Tse HF, Wang CH, Yang NI, Zhou J, Sim D. Understanding the epidemiology of heart failure to improve management practices: an Asia‐Pacific perspective. J Card Fail 2017; 23: 327–339. [DOI] [PubMed] [Google Scholar]
- 4. Son YJ, Kwon BE. Overactive bladder is a distress symptom in heart failure. Int Neurourol J 2018; 22: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu Y, Gupta A, Wu C, Masoudi FA, Du X, Zhang J, Krumholz HM, Li J, China PEACE Collaborative Group . Characteristics, management, and outcomes of patients hospitalized for heart failure in China: the China PEACE retrospective heart failure study. J Am Heart Assoc 2019; 8: e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiba N, Watanabe J, Shinozaki T, Koseki Y, Sakuma M, Kagaya Y, Shirato K, the CHART investigators. Analysis of chronic heart failure registry in the Tohoku district: third year follow‐up. Circ J 2004; 68: 427–434. [DOI] [PubMed] [Google Scholar]
- 7. Lam CS, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto B. Asian sudden cardiac death in heart failure (ASIAN‐HF) registry. Eur J Heart Fail 2013; 15: 928–936. [DOI] [PubMed] [Google Scholar]
- 8. Mentz RJ, Roessig L, Greenberg BH, Sato N, Shinagawa K, Yeo D, Kwok BWK, Reyes EB, Krum H, Pieske B, Greene SJ, Ambrosy AP, Kelly JP, Zannad F, Pitt B, Lam CSP. Heart failure clinical trials in east and southeast Asia: understanding the importance and defining the next steps. JACC Heart Fail 2016; 4: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875–880. [DOI] [PubMed] [Google Scholar]
- 10. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013; 1: 93–102. [DOI] [PubMed] [Google Scholar]
- 11. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all‐cause, cardiovascular, and cancer mortality: sixteen years of follow‐up in US women. Circulation 2008; 117: 1658–1667. [DOI] [PubMed] [Google Scholar]
- 12. Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KA, Smith SC Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C. International day for the evaluation of abdominal obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007; 116: 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gómez‐Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil MJ, Valentí V, Rotellar F, Ramírez B, Salvador J, Frühbeck G. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) (2005) 2012; 36: 286–294. [DOI] [PubMed] [Google Scholar]
- 14. Nevill AM, Stewart AD, Olds T, Holder R. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol 2006; 129: 151–156. [DOI] [PubMed] [Google Scholar]
- 15. Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, Kolonel LN, Murphy SP, Chang L, Novotny R, le Marchand L. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes 2011; 1: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas DM, Bredlau C, Bosy‐Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, Heymsfield SB. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring, Md) 2013; 21: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y, Tao Y, Cheng Y, Liu Y. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross‐sectional study. Diabet Med 2018; 35: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 18. Geraci G, Zammuto M, Gaetani R, Mattina A, D'Ignoto F, Geraci C, Noto D, Averna M, Cottone S, Mulè G. Relationship of a body shape index and body roundness index with carotid atherosclerosis in arterial hypertension. Nutr Metab Cardiovasc Dis: NMCD 2019; 29: 822–829. [DOI] [PubMed] [Google Scholar]
- 19. Yalcin G, Ozsoy E, Karabag T. The relationship of body composition indices with the significance, extension and severity of coronary artery disease. Nutr Metab Cardiovasc Dis: NMCD 2020; 30: 2279–2285. [DOI] [PubMed] [Google Scholar]
- 20. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes 2012; 5: 487–493. [DOI] [PubMed] [Google Scholar]
- 21. Cohen DE, Fisher EA. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis 2013; 33: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong JC, Li J, Pavlova M, Chen S, Wu A, Wu S, Gao X. Risk factors for probable REM sleep behavior disorder: a community‐based study. Neurology 2016; 86: 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, Wang A. Triglyceride‐glucose index is associated with the risk of myocardial infarction: an 11‐year prospective study in the Kailuan cohort. Cardiovasc Diabetol 2021; 20: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Lévy S, Linde C, Lopez‐Sendon JL, Nieminen MS, Piérard L, Remme WJ, Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology . Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005): the task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur Heart J 2005; 26: 1115–1140. [DOI] [PubMed] [Google Scholar]
- 25. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose‐response meta‐analysis of prospective studies. Circulation 2016; 133: 639–649. [DOI] [PubMed] [Google Scholar]
- 26. Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO expert consultation on waist circumference and waist‐hip ratio. Eur J Clin Nutr 2010; 64: 2–5. [DOI] [PubMed] [Google Scholar]
- 27. Ashwell M, Gunn P, Gibson S. Waist‐to‐height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta‐analysis. Obes Rev 2012; 13: 275–286. [DOI] [PubMed] [Google Scholar]
- 28. Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population‐based prospective study. Circ Heart Fail 2009; 2: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist‐to‐hip ratio on the risk of heart failure. Circulation 2010; 121: 237–244. [DOI] [PubMed] [Google Scholar]
- 30. Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc 2006; 54: 413–420. [DOI] [PubMed] [Google Scholar]
- 31. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 2010; 16: e1–e194. [DOI] [PubMed] [Google Scholar]
- 32. Halldin AK, Schaufelberger M, Lernfelt B, Björck L, Rosengren A, Lissner L, Björkelund C. Obesity in middle age increases risk of later heart failure in women‐results from the prospective population study of women and H70 studies in Gothenburg, Sweden. J Card Fail 2017; 23: 363–369. [DOI] [PubMed] [Google Scholar]
- 33. The NS, Richardson AS, Gordon‐Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care 2013; 36: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow‐up of a birth cohort. Diabetes Care 2011; 34: 1986–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norris T, Cole TJ, Bann D, Hamer M, Hardy R, Li L, Ong KK, Ploubidis GB, Viner R, Johnson W. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: a cohort study. PLoS Med 2020; 17: e1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, Zimmer M, Mende M, Linke A, Kiess W, Schuler G, Körner A, Erbs S. Childhood obesity: impact on cardiac geometry and function. J Am Coll Cardiol Img 2014; 7: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 37. Stamler J. Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol 1991; 1: 347–362. [DOI] [PubMed] [Google Scholar]
- 38. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 39. Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012; 366: 54–63. [DOI] [PubMed] [Google Scholar]
- 40. Jacoby RM, Nesto RW. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. J Am Coll Cardiol 1992; 20: 736–744. [DOI] [PubMed] [Google Scholar]
- 41. Raggi P, Cooil B, Ratti C, Callister TQ, Budoff M. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension (Dallas, Tex: 1979) 2005; 46: 238–243. [DOI] [PubMed] [Google Scholar]
- 42. Bahit MC, Kochar A, Granger CB. Post‐myocardial infarction heart failure. JACC Heart Fail 2018; 6: 179–186. [DOI] [PubMed] [Google Scholar]
- 43. Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin‐6 and high sensitivity C‐reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest 2007; 30: 210–214. [DOI] [PubMed] [Google Scholar]
- 44. Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord 1999; 23: 67–74. [DOI] [PubMed] [Google Scholar]
- 45. Messerli FH, Sundgaard‐Riise K, Reisin E, Dreslinski G, Dunn FG, Frohlich E. Disparate cardiovascular effects of obesity and arterial hypertension. Am J Med 1983; 74: 808–812. [DOI] [PubMed] [Google Scholar]
- 46. Engeli S, Sharma AM. The renin‐angiotensin system and natriuretic peptides in obesity‐associated hypertension. J Mol Med (Berl) 2001; 79: 21–29. [DOI] [PubMed] [Google Scholar]
- 47. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A 2000; 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]


