Abstract
Aims
Assessing frailty and sarcopenia is considered a valuable cornerstone of perioperative risk stratification in advanced heart failure patients. The lack of an international consensus on a diagnostic standard impedes its implementation in the clinical routine. This study aimed to compare the feasibility and prognostic impact of different assessment tools in patients undergoing continuous‐flow left ventricular assist device (cf‐LVAD) implantation.
Methods and results
We prospectively compared feasibility and prognostic values of six frailty/sarcopenia assessment methods in 94 patients prior to cf‐LVAD implantation: bioelectrical impedance analysis (BIA), computed tomography (CT)‐based measurement of two muscle areas/body surface area [erector spinae muscle (TMESA/BSA) and iliopsoas muscle (TPA/BSA)], physical performance tests [grip strength, 6 min walk test (6MWT)] and Rockwood Clinical Frailty Scale (RCFS). Six‐month mortality and/or prolonged ventilation time >95 h was defined as the primary endpoint. BIA and CT showed full feasibility (100%); physical performance and RCFS was limited due to patients' clinical status (feasibility: 87% grip strength, 62% 6MWT, 88% RCFS). Phase angle derived by BIA showed the best results regarding the prognostic value for 6 month mortality and/or prolonged ventilation time >95 h (odds ratio (OR) 0.66 [95% confidence interval (CI): 0.46–0.92], P = 0.019; area under the curve (AUC) 0.65). It provided incremental value to the clinical risk assessment of EuroSCORE II: C‐index of the combined model was 0.75 [95% CI; 0.651–0.848] compared with C‐index of EuroSCORE II alone, which was 0.73 (95% CI: 0.633–0.835).
Six‐month survival was decreased in patients with reduced body cell mass derived by BIA or reduced muscle area in the CT scan compared with patients with normal values: body cell mass 65% (95% CI: 51.8–81.6%) vs. 83% (95% CI: 74.0–93.9%); P = 0.03, TMESA/BSA 65% (95% CI: 51.2–82.2%) vs. 82% (95% CI: 73.2–93.0%); P = 0.032 and TPA/BSA 66% (95% CI: 53.7–81.0%) vs. 85% (95% CI: 75.0–95.8%); P = 0.035.
Conclusions
Bioelectrical impedance analysis parameters and CT measurements were shown to be suitable to predict 6‐month mortality and/or prolonged ventilation time >95 h in patients with advanced heart failure prior to cf‐LVAD implantation. Phase angle had the best predictive capacity and sarcopenia diagnosed by reduced body cell mass in BIA or muscle area in CT was associated with a decreased 6 month survival.
Keywords: Ventricular assist device, Frailty evaluation, Sarcopenia, Advanced heart failure, Bioelectrical impedance analysis
Introduction
Identifying patients who are suitable for continuous‐flow left ventricular assist device (cf‐LVAD) implantation remains crucial, especially prior to early implantation [Interagency Registry for Mechanically Assisted Circulatory Support Scale (INTERMACS) level ≥IV] and in view of the rising number of implantations as destination therapy. 1 , 2 A chronological age over 65 years appears to adversely affect the results of cf‐LVAD surgery. 3 , 4 However, the prognostic impact of frailty, as a surrogate for advanced biological age, on the outcome is considered to be superior in cardiac patients. 5 , 6 , 7 Moreover, several trials have identified frailty as an important risk factor for an adverse outcome after cf‐LVAD implantation. 5 , 8 , 9 , 10 Accordingly, the current European Association of Cardiothoracic Surgery (EACTS) expert consensus paper concerning long‐term mechanical circulatory support recommends the evaluation of frailty prior to cf‐LVAD implantation. 11
Frailty is a potentially reversible state characterized by a reduced resilience against stressors due to a multifactorial process resulting in an instability of homoeostasis. 12 , 13 The clinical manifestation resembles symptoms of advanced heart failure (AHF), including exhaustion, weakness and cachexia, which lead to exercise intolerance, sarcopenia and dependency on help. 14 A joint pathological pathway is suspected; therefore, distinguishing frailty from the symptoms of heart failure remains extraordinarily challenging 10 , 15 : Depending on the cohort and the assessment tool used, the estimated prevalence of frailty in advanced heart failure patients varies widely (7–70%) in different studies, but overall appears to be increased compared with the general population. 5 , 9 , 16 , 17
Physicians' options for frailty evaluation include bioelectrical impedance analysis (BIA), image‐supported measurement of muscle areas, physical performance tests and questionnaires. To date, an internationally acknowledged consensus on a diagnostic gold standard is lacking. This hampers the implementation of frailty assessments in the routine evaluation of patients.
Addressing this unmet clinical need, this study was designed to prospectively compare different frailty assessment tools in advanced heart failure patients prior to cf‐LVAD implantation with regard to their feasibility and prognostic impact.
Materials and methods
1. Frailty assessments
a. Bioelectrical impedance analysis
Bioelectrical impedance analysis estimates the body's composition by measuring tissue resistance at different frequencies. While body fluids resemble an ohmic resistance, cells act like a capacitor. The phase angle is calculated from the resulting phase shift between current and voltage in the current circuit. Independently from body weight, it allows for measuring three major prognostic domains 18 : cell integrity as a marker of frailty/biological age, quantitative body cell mass as a surrogate for sarcopenia and fluid balance estimation as an indicator of the decompensation state of heart failure. 19 , 20 , 21 , 22 , 23 , 24 As previously described by Mullie et al., a phase angle ≤4.5° was defined as frail. 24 Body cell mass ≤27 kg, total body water ≥50 L were defined as pathological according to the normal values provided by the BIA device manufacturer Data Input GmbH. 25
We used the portable body composition analyser NUTRIGUARD‐MS (data input GmbH, Germany) for the BIA measurement. The setup was standardized according to the manufacturer's recommendations and the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines. 25 , 26 , 27
b. Image‐based sarcopenia assessments
As a diagnostic tool for sarcopenia, muscle quantity was assessed by measuring the total muscle areas of the erector spinae muscle (TMESA) at the level of thoracic vertebra Th12 and of its physiological antagonist, the iliopsoas muscle (TPA), at the level of lumbar vertebra L4 in a single, axial image of a computed tomography (CT) scan. 28 , 29 Both were indexed for body surface area (BSA) which was calculated using the DuBois formula to balance for body constitution. 30 The threshold for sarcopenia was defined by TMESA/BSA ≤ 17.2 cm2/m2, referring to the results of Minegishi et al. 31 With no comparable cut‐off value in the literature, the TPA/BSA cut‐off was derived empirically from our data with ≤12.5 cm2/m2.
c. Physical performance: grip strength and 6 minute walk test
Muscle quality und functional status were evaluated using a dynamometer (type SAEHAN™, Korea) to measure grip strength. 32 , 33 The mean of three consecutive measurements was calculated. Patients were asked not to rest their arms on their elbows and were allowed to take any position they deemed comfortable. A reduced grip strength dependent on gender and body mass index was defined according to the cut‐off chosen in the fried frailty phenotype and the recommendations of the European consensus on definition and diagnosis of sarcopenia. 12 , 14
A 6 minute walk test (6MWT) was performed according to the guidelines of the American Thoracic Society. 34 , 35 Here, a walking distance ≤300 m, equalling a gait speed below 0.8 m/s as used in the fried frailty phenotype, 12 or inability to complete the started test with a walking time below 5 min was defined as impaired.
d. Rockwood Clinical Frailty Scale
The Rockwood Clinical Frailty Scale is a nine‐step scale that allows physicians to evaluate frailty with regard to patients' deficits, physical activity and their dependence on help to manage their life. 36 Frailty was defined as Classes 5–9 according to Rockwood Clinical Frailty Scale. 36
2. Study design
We prospectively evaluated six frailty/sarcopenia assessments in patients prior to cf‐LVAD implantation.
First, we compared the preoperative feasibility and restrictive factors of the assessments in our cohort.
Second, we calculated the predictive value of frailty test results in two predefined outcome‐related indicator groups: Group A died within 6 months after surgery and/or had a prolonged postoperative mechanical ventilation time >95 h, which has an economic impact according to the DRG (diagnosis‐related groups) system. 37 , 38 , 39 , 40 The combination of 6 month mortality and prolonged ventilation time served as our primary endpoint.
Biermann et al. described the definition of ventilation time according to the DRG system in Germany: a ventilation time >95 h is considered long‐term ventilation. 41 The starting point is the connection to the ventilation machine, independent of the mode. However, in case of intubation within the scope of surgery, time on a ventilation machine is only considered ventilated time if it exceeds 24 h after the end of surgery or if the patients were preoperatively ventilated. 41 Therefore, patients without preoperative ventilation time and who are extubated within 24 h after surgery were recorded with a ventilation time of 0 h. Group B was extubated within 95 h and survived at least 6 months.
In a secondary analysis we evaluated the predictive value of the assessments with respect to 6 month survival alone.
3. Patient cohort, clinical data, and data collection
Frailty assessments were conducted as part of the evaluation process in 94 patients who were referred to our centre for advanced heart failure therapy (cf‐LVAD implantation or heart transplantation). The median time between frailty assessment and surgery, along with a number of measurements, is displayed in Figure 1 . Twenty patients were scheduled for heart transplantation and were initially listed in a ‘high urgent’ status, but underwent emergency cf‐LVAD implantation due to clinical deterioration during the waiting time. Their frailty assessments were carried out at the time of listing in a ‘high urgent’ status for heart transplantation.
Figure 1.
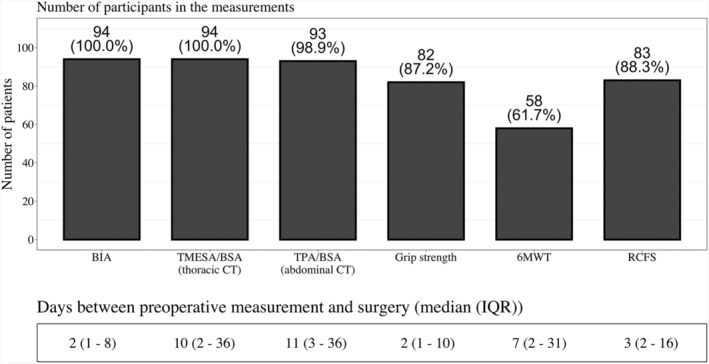
Feasibility of frailty/sarcopenia assessments. Number and percentage of patients, who participated in the measurements and description of time between measurement and cf‐LVAD implantation. Abbreviations: BIA = bioelectrical impedance analysis; TMESA/BSA = total muscle areas of the erector spinae muscle/body surface area; TPA/BSA = total muscle areas of the iliopsoas muscle/body surface area; 6MWT = 6 minute walk test; RCFS = Rockwood Clinical Frailty Scale.
All measurements were conducted by the same specially trained examiner.
Therapeutical decisions were made according to current guidelines/intrahospital standards and were not influenced by the results of the frailty assessments. 11 Due to concerns about exposure to radiation, only clinically indicated CT scans were conducted and analysed. The intrahospital protocol for patient evaluation for advanced heart failure therapies includes a preoperative CT scan to exclude malignancies and current infection as well as surgical planning. The data were collected and managed in a Research Electronic Data Capture platform (REDCap) database. 42 The trial was approved by the ethics committee of the Charité—Universitätsmedizin Berlin (EA2/236/17) and registered online (https://clinicaltrials.gov/) under clinical registration number NCT04222400.
4. Statistical methods
Patients who were not able to perform the frailty assessment were excluded from the affected calculations in the analysis. Ordinal and nominal parameters were described in numbers and percentages, with a χ 2 test performed to compare data between groups. Metric values were analysed using Student's t test or the Mann–Whitney U test, as appropriate. For normally distributed values, the mean value with the standard deviation was indicated; for other distributions, the median with the first and third quartile was declared. A receiver operating characteristic and the area under the curve were (AUC) calculated for each frailty assessment. Univariate logistic regression was calculated to determine the odds ratio (OR). A multivariable logistic regression analysis was conducted to adjust the three most promising frailty/sarcopenia assessments for clinical risk factors represented by EuroSCORE II and C‐indices were compared. Survival of patients with normal test results vs. patients with pathological findings (separately for each assessment) was graphically displayed by Kaplan–Meier curves and compared by log‐rank test.
A P value <0.05 was considered significant. Statistical analysis was performed using the statistics program R Version 3.6.2. All p‐values should be read descriptively.
Results
1. Baseline characteristics
Between April 2018 and February 2019, 110 AHF patients underwent cf‐LVAD implantation in our centre. Ninety‐four (85%) adults were included in this analysis and gave written informed consent. Fifteen patients were not asked due to logistical reasons, for example, patients who were referred from another hospital directly to our operating room, without time for a frailty assessment in between. One patient refused participation. Baseline characteristics are displayed in Table 1 . The level of inotropic support is represented by the inotropic score. 43
Table 1.
Baseline characteristics
| Overall cohort | Group comparison | ||||
|---|---|---|---|---|---|
| Parameter | Level | (N = 94) | Group A (N = 53) | Group B (N = 41) | P value |
| Gender | Female | 10 (10.6%) | 5 (9.4%) | 5 (12.2%) | 0.926 |
| Male | 84 (89.4%) | 48 (90.6%) | 36 (87.8%) | ||
| Age (years) | 59.00 [53.25, 65.00] | 61.00 [55.00, 66.00] | 58.00 [53.00, 63.00] | 0.087 | |
| Weight (kg) | 87.05 [76.15, 99.28] | 88.70 [76.00, 103.00] | 85.80 [77.30, 95.00] | 0.617 | |
| Height (m) | 1.78 (0.08) | 1.76 (0.08) | 1.80 (0.08) | 0.076 | |
| Body surface (m2) | 2.07 (0.20) | 2.07 (0.23) | 2.07 (0.17) | 0.953 | |
| BMI (kg/m2) | 27.50 [25.00, 32.00] | 29.00 [25.00, 33.00] | 27.00 [25.00, 30.00] | 0.208 | |
| Disease | CAD | 49 (52.1%) | 33 (62.3%) | 16 (39.0%) | 0.036 |
| DCMP | 41 (43.6%) | 17 (32.1%) | 24 (58.5%) | ||
| Other | 4 (4.3%) | 3 (5.7%) | 1 (2.4%) | ||
| NYHA | II | 1 (1.1%) | 0 (0.0%) | 1 (2.4%) | 0.21 |
| III | 28 (29.8%) | 13 (24.5%) | 15 (36.6%) | ||
| IV | 65 (69.1%) | 40 (75.5%) | 25 (61.0%) | ||
| INTERMACS | I | 20 (21.3%) | 15 (28.3%) | 5 (12.2%) | 0.262 |
| II | 33 (35.1%) | 18 (34.0%) | 15 (36.6%) | ||
| III | 15 (16.0%) | 8 (15.1%) | 7 (17.1%) | ||
| IV | 25 (26.6%) | 11 (20.8%) | 14 (34.1%) | ||
| VI | 1 (1.1%) | 1 (1.9%) | 0 (0.0%) | ||
| EuroSCORE II (%) | 17.87 [9.17, 29.04] | 23.48 [14.32, 39.87] | 13.05 [6.57, 18.67] | <0.001 | |
| Inotropic score | 8.29 [4.76, 17.44] | 8.50 [5.25, 19.87] | 6.08 [4.47, 15.60] | 0.147 | |
| cf‐LVAD | HeartMate III | 21 (22.3%) | 12 (22.6%) | 9 (22.0%) | 1.000 |
| HeartWare | 73 (77.7%) | 41 (77.4%) | 32 (78.0%) | ||
| Haemoglobin (g/dL) | 10.80 [9.30, 12.70] | 10.60 [8.60, 12.00] | 11.65 [10.12, 12.93] | 0.043 | |
| Haematocrit (%) | 33.20 [28.50, 38.40] | 31.70 [26.30, 38.00] | 35.10 [31.17, 39.08] | 0.068 | |
| Creatinine (mg/dL) | 1.40 [1.00, 1.90] | 1.60 [1.20, 2.10] | 1.30 [1.00, 1.63] | 0.063 | |
| Albumin (g/dL) | 3.10 [2.50, 3.50] | 2.70 [2.10, 3.42] | 3.40 [3.00, 3.60] | 0.003 | |
| CRP (mg/dL) | 2.80 [1.00, 8.50] | 4.30 [1.40, 10.40] | 1.60 [0.48, 4.62] | 0.011 | |
| Bilirubin (mg/dL) | 1.00 [0.68, 1.70] | 1.00 [0.73, 1.70] | 0.96 [0.66, 1.70] | 0.519 | |
| NT‐proBNP (pg/dL) | 10,669.51(10,628.35) | 12,395.49 (12,677.16) | 8,766.51 (7,488.13) | 0.123 | |
| Lactate (mg/dL) | 9.00 [6.00, 12.00] | 9.50 [7.75, 12.25] | 6.00 [5.00, 10.50] | 0.005 | |
| 6 month mortality | Yes | 23 (24.5%) | 23 (43.4%) | 0 (0.0%) | |
| No | 71 (75.5%) | 30 (56.6%) | 41 (100.0%) | ||
| Survival time (days) | 614 [286, 734] | 477 [66, 644] | 716 [596, 763] | <0.001 | |
| Length of ICU stay (days) | 17.50 [7.00, 40.25] | 33.50 [14.50, 55.50] | 7.00 [4.75, 15.50] | <0.001 | |
| Length of stay (days) | 45.00 [28.25, 82.75] | 58.00 [35.00, 107.00] | 36.00 [26.00, 58.00] | 0.006 | |
| Ventilation time (hours) | 106.50 [22.75, 626.25] | 567.00 [239.00, 943.00] | 22.00 [0.00, 34.00] | ||
Baseline characteristics of all patients and comparison of patients with ventilation time >95 h and/or death within 6 months of surgery (Group A) vs. patients with ventilation time <95 h and minimum survival of 6 months after surgery (Group B), Values are stated as number (%), mean (standard deviation) or median [interquartile range], and groups were compared with Student's t test, Mann–Whitney U test or χ 2 test as appropriate.
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DCMP, dilated cardiomyopathy.
Group A (adverse outcome) included 53 (56%) patients: the overall 6 month mortality was 25%, and 48 (51%) patients needed prolonged mechanical ventilation for more than 95 h. Eighteen (38%) of the patients with prolonged ventilation died within a period of 6 months.
2. Feasibility of the frailty assessments
No serious adverse events occurred during or after the measurements. BIA and CT were available without limitation, although one scan did not include level L4. Grip strength was conducted in 82 (87%) patients and was limited mainly due to cardiopulmonary instability (INTERMACS 1&2 or short‐term circulatory support) (n = 12; 13%). Limiting factors for the availability of the 6MWT in 36 (38%) cases were low central venous O2 saturation, haemodynamic instability despite inotropic support or preoperative treatment in the intensive care unit, including short‐term circulatory support (n = 30; 32%) and sedation/preoperative mechanical ventilation alone (n = 3; 3%). Symptoms most commonly reported by patients during the 6MWT were shortness of breath, stable angina pectoris, weakness and orthopaedic problems; in 3 (3%) patients these symptoms were so strong that they were not able to proceed with the 6MWT. Conducting the Rockwood Clinical Frailty Scale was not possible in 11 patients (Figure 1 ).
3. Comparison of the impact of frailty assessments regarding the combined endpoint (6 month mortality and/or prolonged ventilation)
Group A patients had a significantly lower phase angle compared with those in group B. Further analysis of BIA parameters showed a trend towards higher total body water and extracellular water in patients of group A, whereas intracellular water and body cell mass did not differ significantly between the two groups. All other frailty/sarcopenia assessments showed no significant differences between the two groups (Table 2 : frailty/sarcopenia assessments). The phase angle was shown to have an acceptable predictive power for 6 month mortality and/or prolonged mechanical ventilation (AUC 0.65 [95% CI: 0.535–0.758]), whereas all other frailty/sarcopenia assessments failed to exhibit a predictive power (AUC < 0.60).
Table 2.
Frailty assessments
| Parameter |
Overall cohort N = 94 |
Group comparison | P value | |||
|---|---|---|---|---|---|---|
| Group A | Group B | |||||
| Phase angle (°) | 4.10 [3.20, 4.97] | N = 53 | 3.70 [3.00, 4.70] | N = 41 | 4.30 [3.90, 5.10] | 0.015 |
| Body water (L) | 52.50 [44.67, 58.57] | 54.10 [45.20, 60.30] | 51.00 [43.50, 54.40] | 0.061 | ||
| Extracellular water (L) | 24.45 [19.52, 29.17] | 25.60 [19.60, 30.70] | 22.60 [19.50, 25.60] | 0.037 | ||
| Intracellular water (L) | 28.10 [25.15, 29.87] | 28.00 [25.10, 31.00] | 28.50 [25.30, 29.30] | 0.356 | ||
| Body cell mass (kg) | 27.95 [23.83, 33.10] | 27.10 [23.70, 33.20] | 28.90 [24.00, 32.80] | 0.437 | ||
| TMESA/BSA (cm2/m2) | 18.68 [15.44, 21.04] | N = 53 | 18.34 [14.87, 21.26] | N = 41 | 18.90 [16.11, 20.75] | 0.522 |
| TPA/BSA (cm2/m2) | 12.49 [10.67, 14.02] | N = 52 | 12.16 [10.50, 13.46] | N = 41 | 12.86 [10.91, 14.91] | 0.132 |
| Grip strength (kg) | 30.00 [24.00, 39.00] | N = 41 | 29.00 [24.00, 38.00] | N = 41 | 31.00 [25.00, 39.00] | 0.430 |
| Grip strength/weight (%) | 33.70 [27.43, 43.93] | 32.63 [25.05, 41.18] | 36.50 [29.55, 48.48] | 0.196 | ||
| Walking distance (m) | 274.00 [170.50, 347.75] | N = 27 | 282.00 [184.00, 349.50] | N = 31 | 255.00 [154.50, 341.00] | 0.518 |
| Rockwood Clinical Frailty Scale | N = 42 | N = 41 | 0.194 | |||
| 2‐ Well | 1 (1.2%) | 0 (0.0%) | 1 (2.4%) | |||
| 3‐ Managing well | 3 (3.6%) | 1 (2.4%) | 2 (4.9%) | |||
| 4‐Vulnerable | 19 (22.9%) | 10 (23.8%) | 9 (22.0%) | |||
| 5‐Mildly frail | 19 (22.9%) | 8 (19.0%) | 11 (26.8%) | |||
| 6‐Moderately frail | 20 (24.1%) | 12 (28.6%) | 8 (19.5%) | |||
| 7‐Severely frail | 16 (19.3%) | 6 (14.3%) | 10 (24.4%) | |||
| 8‐Very severely frail | 5 (6.0%) | 5 (11.9%) | 0 (0.0%) | |||
Results of the frailty/sarcopenia measurements for all patients and patients with a ventilation time >95 h and/or death within 6 months after surgery (Group A) vs. patients with ventilation time <95 h and minimum survival of 6 months after surgery (Group B). Values are stated as numbers (%) or median [interquartile range], and groups were compared with Mann–Whitney U test or χ 2 test as appropriate.
Abbreviations: TMESA/BSA, total m. erector spinae muscle area/body surface area; TPA/BSA, total area of m. iliopsoas/body surface area.
The risk of belonging to Group A was reduced by 44% per 1° increase in phase angle. The groups showed a significant increase in the risk for an adverse outcome per increase in body water (4% per 1 L) and extracellular water (8% per 1 L). There was no significantly increased risk of belonging to Group A by a decrease in body cell mass, muscle mass or function (Figure 2 ).
Figure 2.
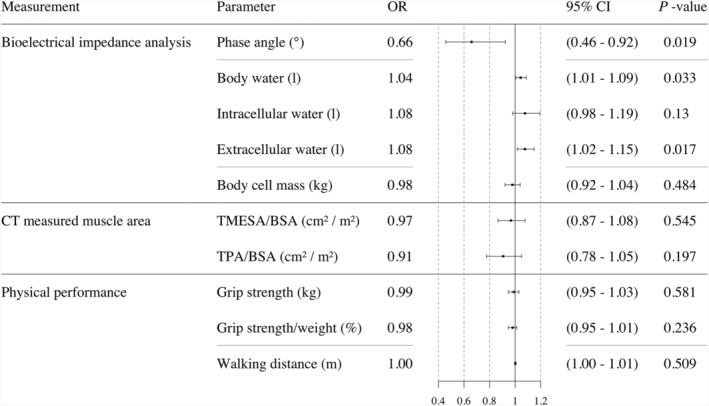
Univariable logistic regression analysis. Odds ratio of frailty/sarcopenia assessments for the endpoint 6 month mortality and/or ventilation time >95 h. Abbreviations: CT = computed tomography; TMESA/BSA = total muscle areas of the erector spinae muscle/body surface area; TPA/BSA = total muscle areas of the iliopsoas muscle/body surface area.
Phase angle was not independently significant after adjusting for clinical risk; however, adding phase angle to established clinical risk factors represented by EuroSCORE II did increase the discriminating power of the risk estimation: the C‐index of the combined model was 0.75 [95% confidence interval (CI): 0.651–0.848) compared with EuroSCORE II alone [C‐index 0.73 (95% CI: 0.633–0.835)]. TPA/BSA showed a trend towards significance; the combined model had the highest discriminating power for the combined endpoint [C‐index 0.751 (95% CI: 0.652–0.850)] (see Figure 3 ).
Figure 3.
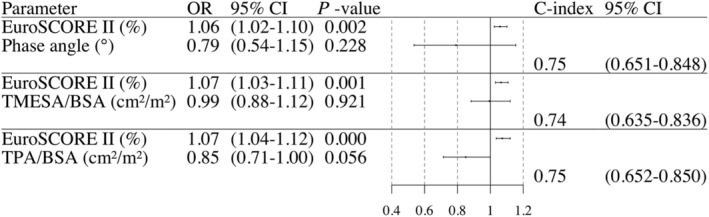
Multivariable logistic regression analysis. Odds ratio and statement of the C‐index of frailty/sarcopenia assessments adjusted for clinical risk represented by EuroSCORE II in a multivariable logistic regression analysis for the endpoint 6‐month mortality and/or ventilation time >95 h. Abbreviations: TMESA/BSA = total muscle areas of the erector spinae muscle/body surface area; TPA/BSA = total muscle areas of the iliopsoas muscle/body surface area.
4. Kaplan–Meier analysis for 6 month survival
The 6 month survival of the overall cohort was 75% (95% CI: 67.3–84.7%).
Reduced muscle mass, represented by body cell mass [65% (95% CI: 51.7–81.6%) vs. 83% (95% CI: 74.0–93.9%); P = 0.03] or reduced muscle area in the CT measurement [TMESA/BSA 65% (95% CI: 51.2–82.2%) vs. 82% (95% CI: 73.2–93.0%); P = 0.032, and TPA/BSA 66% (95% CI: 53.7–81.0%) vs. 85% (95% CI: 75.0–95.8%); P = 0.035] were associated with a reduced 6 month survival compared with normal muscle values, whereas all other measurement were not; see Figure 4 .
Figure 4.
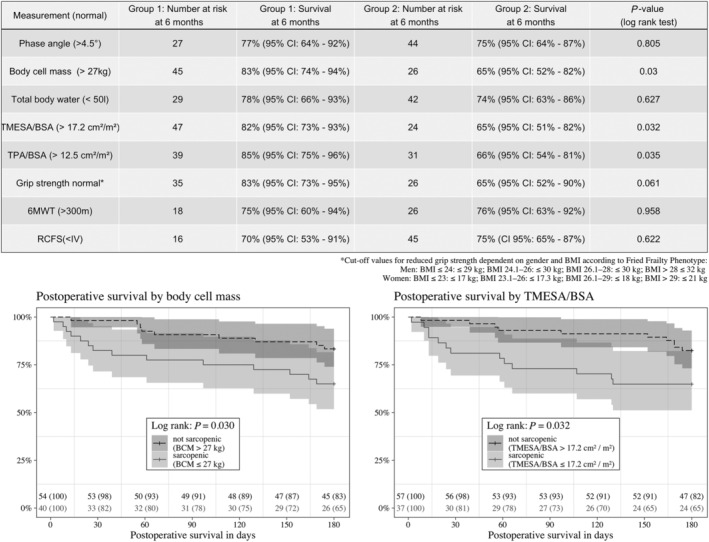
Six‐month survival—patients with normal measurement results (Group 1) vs. patient with reduced results (Group 2). Abbreviations: BCM = body cell mass; TMESA/BSA = total muscle areas of the erector spinae muscle/body surface area; TPA/BSA = total muscle areas of the iliopsoas muscle/body surface area; 6MWT = 6 min walk test; RCFS = Rockwood Clinical Frailty Scale.
Discussion
Frailty assessments in advanced heart failure patients have several limitations attributable to their failure to discriminate between frailty and heart failure symptoms. 10 , 15 , 44 , 45 An acknowledged gold standard for diagnosis is not yet available and validated cut‐off values remain scarce for most measurements, especially in terminally ill patients.
One of the most widely used assessments is the Fried Frailty Phenotype developed by Fried et al. 12 Jha et al. used a modified version of this assessment to phenotype their advanced heart failure cohort prior to heart transplantation, and found a prevalence of frailty in 33% of their patients with an association between increased postoperative mortality and frailty. 46 They found also an association between frailty and NYHA class, highlighting the overlap between heart failure symptoms and frailty. 46 Accordingly, the ESC/HFA position paper on frailty in advanced heart failure patients discusses advantages and limitations of the fried frailty phenotype and its single‐item components, concluding that a tailored assessment tool is necessary for advanced heart failure patients. 10
Therefore, we omitted self‐reported exhaustion and low physical activity due to the obvious difficulties of distinguishing these parameters, especially in advanced heart failure patients. We included BIA and estimation of the muscle areas in a CT as two objective measurements of muscle mass. The question about unintentional weight loss was abandoned, because loss of body weight caused by sarcopenia may be masked by oedema or induced by the use of diuretics in advanced heart failure patients. We extracted the physical performance assessment by estimating the walking ability and grip strength from the fried frailty phenotype for our analysis. We also included the Rockwood Clinical Frailty Scale.
Most frailty tools including the fried frailty phenotype are validated for patients aged >65 years, 12 while most patients undergoing cf‐LVAD implantation are younger. In large registries, 60% of patients undergoing cf‐LVAD implantation are aged 50–60 years and only 12% are older than 70 years. 47 In our cohort, 73% of the patients were younger than 65 years.
Patient‐centred outcomes, such as postsurgical quality of life and physical abilities after cf‐LVAD implantation may also indicate a successful surgery, along with high survival rates. 20% of patients report a reduced quality of life after cf‐LVAD implantation. 2 Prolonged postoperative ventilation is associated not only with higher mortality, but also with long‐term adverse outcomes like critical illness polyneuropathy and myopathy, infections and psychological trauma. 48 Economic parameters are gaining importance as the costs of our health care system rise. Ventilation weaning, especially after prolonged ventilation, is highly dependent on muscle function; therefore, we assume a direct connection between frailty/sarcopenia and the need for prolonged ventilation.
1. Feasibility of the frailty assessments
Of all evaluated methods, we were able to perform BIA in 100% of patients: Independently from active participation and exercise tolerance, it can be performed at the bedside with minimal time expenditure and no known negative side effects. 49 CT showed a comparable availability, but required a greater logistical effort, especially in sedated patients. In contrast to BIA, its usefulness for subsequent measurements for monitoring progression of frailty is limited due to the side effects of the radiation. If CT scans are performed as a routine evaluation tool for cf‐LVAD implantation, it is important for the protocol to be equivalent. In our cohort, CT was not repeated if images were available from a CT scan performed in the 12 months before; therefore, perfect comparability was not given. In this situation, a CT scan may be of only limited value for assessing frailty and the durability of muscle mass measurements needs to be further explored, because short‐term changes in muscle mass may not be represented in older scans. Additionally, sicker patients tend to have multiple and more recent CT scans available.
Because our cohort included patients across all INTERMACS levels, physical performance was not available for every patient. Furthermore, heart failure symptoms limited patients' physical activity, including the measurement thereof. Similarly, in their retrospective analysis of INTERMACS registry data Cooper et al. reported that 42% of patients were too sick to perform the 6MWT prior to cf‐LVAD implantation, which is consistent with our findings. 50 Joseph et al. reported equivalent results in their cohort of 75 prospective LVAD patients: 41% of patients were not able to proceed with the 5 m gait speed test. 8 According to the current INTERMACS report more than 50% of LVAD patients are reported as being in INTERMACS Level I and II prior to implantation. 51 Therefore, we regard availability of the assessment tool even in the most severely ill patients as absolutely essential.
2. Outcome evaluation
a. Bioelectrical impedance analysis
In our cohort, phase angle showed the best predictive value regarding our primary endpoint compared with the other methods assessed, and patients with a lower body cell mass had a significantly lower 6 month survival: Lower phase angle, which is influenced by body water and cell mass, was associated with the endpoint and the risk of an adverse outcome increased by 44% per decreased degree in phase angle. Phase angle increased the discriminating power of established risk factors, represented here by EuroSCORE II, in the combined model for the combined endpoint. Mullie et al. described an association between lower phase angle and frailty diagnosed by the Short Physical Performance Battery and the fried frailty phenotype in cardiosurgical patients. 24 Higher body water in patients with adverse outcomes may indicate a reduced cell quality as a surrogate for frailty and/or higher congestion. To differentiate the impact of congestion on the phase angle from the influence of frailty on the phase angle, sequential measurements with a comparison to development of body weight, oedema, and muscle mass over a time period should be part of further research.
b. Computed tomography‐based evaluation of the muscle areas
TMESA/BSA and TPA/BSA showed no predictive value for the combined endpoint of 6 month mortality and/or prolonged ventilation time >95 h, but patients with a lower muscle area of both core muscles exhibited a significantly worse 6 month survival. To the best of our knowledge, this is the first analysis of TMESA/BSA in the context of cf‐LVAD implantation; however, Minegishi et al. reported an association between TMESA/BSA and an unfavourable outcome after pneumonia. 31 Miller et al. found an increased 30 day mortality or prolonged hospital stay in patients with a reduced TMESA area standardized for body height after lobectomy. 28
In combination with a clinical risk assessment, TPA/BSA showed a trend towards significance and increased the discriminating power of EuroSCORE II. The impact of sarcopenia diagnosed by TPA in patients after cf‐LVAD implantation on prolonged hospital stay or inpatient death was previously described by Heberton et al. 29 ; however, they could not find a significant difference in the overall 3 year mortality. Their measurement modalities differed slightly from ours, therefore, we were unable to use their cut‐off value, but their results on a suitable cut‐off were comparable with ours (12.0 cm2/m2 for males vs 12.5 cm2/m2 in our mostly male cohort).
Calculations regarding muscle density, which could provide more information about the fat and water content in the muscles, were limited due to the difference in contrast agent utilization in our cohort and alterations in contrast agent travel time due to the impaired cardiac output, which allows no appropriate adjustment for these confounders. 52
c. Physical performance tests
Physical performance is reported to be impaired in AHF patients due to a floor effect caused by the nature of the disease. 8 , 10 Joseph et al. studied the predictive value of grip strength prior to cf‐VAD implantation in 75 patients; however, they too were unable to find an association between in‐hospital death and prolonged hospital stay or ventilation time and grip strength. 8 In accordance with these findings reduced grip strength did not reach significance with respect to the combined endpoint, nor with respect to 6 month survival in our cohort.
In their retrospective analysis of INTERMACS registry data, Cooper et al. confirmed our findings of a lack of difference in 1 year mortality regarding the gait speed or the 6 min walk distance. 50 Joseph et al. reported the same shortcomings in the prognostic value for the 5 m gait speed test. 8 Although physical exercise including walking is encouraged in patients on short‐term circulatory support, a performance evaluation would not yield reliable results for muscle quality. The influence of positive inotropic support on the results of physical performance tests and on the validity of frailty assessments in cardiogenic shock patients is still unclear. Physical performance estimated by a walking test was not a suitable assessment tool in our advanced heart failure cohort due to its limited availability and impaired prognostic value possibly caused by the overlap of heart failure symptoms and frailty.
d. Rockwood Clinical Frailty Scale
In our study, the Rockwood Clinical Frailty Scale failed to discriminate between frailty and heart failure symptoms. By definition, all patients with end‐stage heart failure are life‐threateningly ill and approaching the end of their life. Despite that, with regard to managing activities of daily living, most patients were between Rockwood 4–6. The prognostic impact was poor, which confirms the need for a more objective and specific measurement.
3. Study limitations
First, our single‐centre pilot study was conducted unblinded. With ventilation time and 6 month mortality, we chose a rather short‐term outcome. The background noise of the baseline surgical risk may have reduced the impact of frailty/sarcopenia on the outcome in this small cohort. Additionally, the full impact of frailty/sarcopenia might only become apparent in the long‐term outcome of these patients. Therefore, even though we were able to compare the different frailty assessment methods, the overall impact of frailty/sarcopenia—regardless of the method—was poor and we were unable to reproduce the results of other research groups.
INTERMACS Levels I–VI were represented, including 71% of patients on short‐term circulatory support or positive inotropic support. Therefore, not every frailty assessment tool was available in every patient, which limited the number of patients.
Our trial focused on potential evaluation methods of the clinical and functional component and to a large extent neglected the social and psycho‐cognitive domain of frailty, because assessments of these domains are already implemented in the routine evaluation of patients prior to cf‐LVAD implantation. 11
With only 10% female patients in our already small cohort, we waived gender‐based adjustments.
Due to the small sample size, it was not possible to perform a multivariable analysis adjusting for more than one variable.
Conclusions
Frailty evaluation in AHF patients remains extraordinarily challenging and a tailored assessment is necessary for its implementation in routine clinical evaluations. BIA was superior to all other assessment tools in our study with respect to feasibility, logistics and predictive value.
Evaluation of muscle area via CT was feasible in our cohort and able to predict 6 month survival, but is associated with well‐known restrictions like exposure to radiation and consumption of resources.
Physical performance tests and the Rockwood Clinical Frailty Scale were of limited availability in advanced heart failure patients and failed to discriminate between heart failure and frailty.
Funding statement
This project was kindly supported by the personal research grant ‘Kaltenbach Doktoranden‐Stipendium’ from the German Heart Foundation to Luise Roehrich (project number: K/38/18).
Open‐access funding enabled and organized by Projekt DEAL.
Conflict of interest
Prof Dr Falk reports grants from Medtronic GmbH, Abbott GmbH & Co. KG, Boston Scientific, Edwards Lifesciences, JOTEC/CryoLife and other financial activities from Berlin Heart, Biotronik SE & Co., Novartis Pharma GmbH, Zurich Heart outside of the submitted work.
Dr Schoenrath reports other financial activities from Novartis, Abbott, Orion Pharma, AstraZeneca and non‐financial support from Medtronic outside of the submitted work.
Ms Roehrich reports grants from the German Heart Foundation during the conduct of the study. Share holdings of Alianz SE, Carl Zeiss Meditec AG, CompuGroup Medical SE & Co. KGaA, Evotec SE, Fresenius Medical Care AG & Co. KGaA outside of the submitted work.
Nothing to disclose for the other authors.
Roehrich, L. , Sündermann, S. H. , Just, I. A. , Kopp Fernandes, L. , Stein, J. , Solowjowa, N. , Mulzer, J. , Mueller, M. , Hummel, M. , Knierim, J. , Potapov, E. , Falk, V. , and Schoenrath, F. (2022) Comparison of feasibility and results of frailty assessment methods prior to left ventricular assist device implantation. ESC Heart Failure, 9: 1038–1049. 10.1002/ehf2.13764.
Clinical registration number: NCT04222400.
Number of ethics approval: EA2/236/17.
References
- 1. Starling RC, Estep JD, Horstmanshof DA, Milano CA, Stehlik J, Shah KB, Bruckner BA, Lee S, Long JW, Selzman CH, Kasirajan V, Haas DC, Boyle AJ, Chuang J, Farrar DJ, Rogers JG, ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2‐year results. JACC Heart Fail 2017; 5: 518–527. [DOI] [PubMed] [Google Scholar]
- 2. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 3. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015; 34: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 4. Caraballo C, DeFilippis EM, Nakagawa S, Ravindra NG, Miller PE, Mezzacappa C, McCullough M, Gruen J, Levin A, Reinhardt S, Mullan C, Ali A, Maurer MS, Desai NR, Ahmad T, Topkara VK. Clinical outcomes after left ventricular assist device implantation in older adults: an INTERMACS analysis. JACC Heart failure 2019; 7: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 5. Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, Roger VL, Kushwaha SS. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant 2014; 33: 359–365 Epub 2013 Dec 27. PMID: 24486165; PMCID: PMC3966938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chikwe J, Adams DH. Frailty: the missing element in predicting operative mortality. Semin Thorac Cardiovasc Surg 2010; 22: 109–110. [DOI] [PubMed] [Google Scholar]
- 7. Furukawa H, Tanemoto K. Frailty in cardiothoracic surgery: systematic review of the literature. Gen Thorac Cardiovasc Surg 2015; 63: 425–433 Epub 2015 Apr 28. PMID: 25916404. [DOI] [PubMed] [Google Scholar]
- 8. Joseph SM, Manghelli JL, Vader JM, Keeney T, Novak EL, Felius J, Martinez SC, Nassif ME, Lima B, Silvestry SC, Rich MW. Prospective assessment of frailty using the fried criteria in patients undergoing left ventricular assist device therapy. Am J Cardiol 2017; 120: 1349–1354 Epub 2017 Aug 1. PMID: 28843393. [DOI] [PubMed] [Google Scholar]
- 9. Tse G, Gong M, Wong SH, Wu W, Bazoukis G, Lampropoulos K, Wong WT, Xia Y, Wong M, Liu T, Woo J, International Health Informatics Study (IHIS) Network . Frailty and clinical outcomes in advanced heart failure patients undergoing left ventricular assist device implantation: a systematic review and meta‐analysis. J Am Med Dir Assoc 2018; 19: 255–261.e1. [DOI] [PubMed] [Google Scholar]
- 10. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano G, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 11. Potapov EV, Antonides C, Crespo‐Leiro MG, Combes A, Färber G, Hannan MM, Kukucka M, de Jonge N, Loforte A, Lund LH, Mohacsi P, Morshuis M, Netuka I, Özbaran M, Pappalardo F, Scandroglio AM, Schweiger M, Tsui S, Zimpfer D, Gustafsson F. 2019 EACTS expert consensus on long‐term mechanical circulatory support. Eur J Cardiothorac Surg 2019; 56: 230–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 13. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xue QL, Varadhan R. What is missing in the validation of frailty instruments? J Am Med Dir Assoc 2014; 15: 141–142. [DOI] [PubMed] [Google Scholar]
- 16. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2017; 236: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNallan SM, Chamberlain AM, Gerber Y, Singh M, Kane RL, Weston SA, Dunlay SM, Jiang R, Roger VL. Measuring frailty in heart failure: a community perspective. Am Heart J 2013; 166: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 2017; 8: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilic MK, Kizilarslanoglu MC, Arik G, Bolayir B, Kara O, Dogan Varan H, Sumer F, Kuyumcu ME, Halil M, Ulger Z. Association of bioelectrical impedance analysis‐derived phase angle and sarcopenia in older adults. Nutr Clin Pract 2017; 32: 103–109. [DOI] [PubMed] [Google Scholar]
- 21. Bansal N, Zelnick LR, Himmelfarb J, Chertow GM. Bioelectrical impedance analysis measures and clinical outcomes in CKD. Am J Kidney Dis 2018; 72: 662–672. [DOI] [PubMed] [Google Scholar]
- 22. González‐Islas D, Arámbula‐Garza E, Orea‐Tejeda A, Castillo‐Martínez L, Keirns‐Davies C, Salgado‐Fernández F, Hernández‐Urquieta L, Hernández‐López S, Pilotzi‐Montiel Y. Body composition changes assessment by bioelectrical impedance vectorial analysis in right heart failure and left heart failure. Heart Lung 2020; 49: 42–47. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka S, Ando K, Kobayashi K, Seki T, Hamada T, Machino M, Ota K, Morozumi M, Kanbara S, Ito S, Ishiguro N, Hasegawa Y, Imagama S. Low bioelectrical impedance phase angle is a significant risk factor for frailty. Biomed Res Int 2019; 2019: 6283153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullie L, Obrand A, Bendayan M, Trnkus A, Ouimet MC, Moss E, Chen‐Tournoux A, Rudski LG, Afilalo J. Phase angle as a biomarker for frailty and postoperative mortality: the BICS study. J Am Heart Assoc 2018; 7: e008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Data Input GmbH . Das B.I.A.‐Kompendium 3. Ausgabe. 3rd ed. Data Input GmbH 2005. German.
- 26. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent‐Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C, Composition of the ESPEN Working Group . Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr (Edinburgh, Scotland) 2004; 23: 1226–1243. [DOI] [PubMed] [Google Scholar]
- 27. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent‐Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AMWJ, Pichard C, ESPEN . Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr (Edinburgh, Scotland) 2004; 23: 1430–1453. [DOI] [PubMed] [Google Scholar]
- 28. Miller JA, Harris K, Roche C, Dhillon S, Battoo A, Demmy T, Nwogu CE, Dexter EU, Hennon M, Picone A, Attwood K, Yendamuri S. Sarcopenia is a predictor of outcomes after lobectomy. J Thorac Dis 2018; 10: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heberton GA, Nassif M, Bierhals A, Novak E, LaRue SJ, Lima B, Hall S, Silvestry S, Joseph SM. Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol 2016; 118: 1363–1367. [DOI] [PubMed] [Google Scholar]
- 30. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition (Burbank, Los Angeles County, Calif) 1989; 5: 303–313. [PubMed] [Google Scholar]
- 31. Minegishi Y, Inoue S, Sato K, Abe K, Murano H, Furuyama K, Yang S, Machida H, Nakano H, Sato M, Nemoto T, Sato C, Nishiwaki M, Kimura T, Yamauchi K, Igarashi A, Tokairin Y, Shibata Y, Watanabe M. Smaller erector spinae muscle size is associated with inability to recover activities of daily living after pneumonia treatment. Respir Investig 2019; 57: 191–197 Epub 2018 Dec 11. PMID: 30552073. [DOI] [PubMed] [Google Scholar]
- 32. Chung CJ, Wu C, Jones M, Kato TS, Dam TT, Givens RC, Templeton DL, Maurer MS, Naka Y, Takayama H, Mancini DM, Schulze PC. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J Card Fail 2014; 20: 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. SAEHAN™ grip strength dynamometer (Korea). http://saehanmedical.com/sub/eng/product/product_evaluation.html, (16 January 2021)
- 34. Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, Gensini GF. Prognostic value of 6‐minute walk corridor test in patients with mild to moderate heart failure: comparison with other methods of functional evaluation. Eur J Heart Fail 2003; 5: 247–252. [DOI] [PubMed] [Google Scholar]
- 35. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 36. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. InEK, Fallpauschalenkatalog—G‐DRG‐System 2019. https://www.g‐drg.de/G‐DRG‐System_2019/Fallpauschalen‐Katalog/Fallpauschalen‐Katalog_2019, (31/7/2019)
- 38. Kou HW, Yeh CH, Tsai HI, Hsu CC, Hsieh YC, Chen WT, Cheng HT, Yu MC, Lee CW. Sarcopenia is an effective predictor of difficult‐to‐wean and mortality among critically ill surgical patients. PLoS ONE 2019; 14: e0220699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Akyar S, Armenia SJ, Ratnani P, Merchant AM. The impact of frailty on postoperative cardiopulmonary complications in the emergency general surgery population. Surg J (New York, NY) 2018; 4: e66–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papathanasiou M, Mincu RI, Lortz J, Horacek M, Koch A, Pizanis N, Kamler M, Rassaf T, Luedike P. Prolonged mechanical ventilation after left ventricular assist device implantation: risk factors and clinical implications. ESC Heart Fail 2019; 6: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Biermann A, Geissler A. Beatmungsfälle und Beatmungsdauer in deutschen krankenhäusern, Edition: 1 ed. German: Universitätsverlag der TU Berlin. ISBN: 978‐3‐7983‐2631‐6; 2014. [Google Scholar]
- 42. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belletti A, Jacobs S, Affronti G, Mladenow A, Landoni G, Falk V, Schoenrath F. Incidence and predictors of postoperative need for high‐dose inotropic support in patients undergoing cardiac surgery for infective endocarditis. J Cardiothorac Vasc Anesth 2018; 32: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 44. Ayesta A, Astiz M, Masa M, Segovia J, Cosío M, Martínez‐Sellés M. Rationale and design of the FELICITAR registry (Frailty Evaluation After List Inclusion, Characteristics and Influence on Transplantation and Results). Clin Cardiol 2018; 41: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pugh RJ, Ellison A, Pye K, Subbe CP, Thorpe CM, Lone NI, Clegg A. Feasibility and reliability of frailty assessment in the critically ill: a systematic review. Crit Care 2018; 22: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jha SR, Hannu MK, Chang S, Montgomery E, Harkess M, Wilhelm K, Hayward CS, Jabbour A, Spratt PM, Newton P, Davidson PM, Macdonald PS. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation 2016; 100: 429–436 PMID: 26516676. [DOI] [PubMed] [Google Scholar]
- 47. Kirklin JK, Xie R, Cowger J, de By T, Nakatani T, Schueler S, Taylor R, Lannon J, Mohacsi P, Gummert J, Goldstein D, Caliskan K, Hannan MM. Second annual report from the ISHLT mechanically assisted circulatory support registry. J Heart Lung Transplant 2018; 37: 685–691. [DOI] [PubMed] [Google Scholar]
- 48. Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long‐term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta‐analysis. Lancet Respir Med 2015; 3: 544–553. [DOI] [PubMed] [Google Scholar]
- 49. Roehrich L, Suendermann S, Just IA, Knierim J, Mulzer J, Mueller M, Eulert‐Grehn JJ, Hummel M, Starck C, Potapov E, Knosalla C, Falk V, Schoenrath F. Safety of bioelectrical impedance analysis in advanced heart failure patients. Pacing Clin Electrophysiol: PACE 2020; 43: 1078–1085. [DOI] [PubMed] [Google Scholar]
- 50. Cooper LB, Hammill BG, Allen LA, Lindenfeld J, Mentz RJ, Rogers JG, Milano CA, Patel CB, Alexander KP, Hernandez AF. Assessing frailty in patients undergoing destination therapy left ventricular assist device: observations from interagency registry for mechanically assisted circulatory support. ASAIO J 2018; 64: 16–23. [DOI] [PubMed] [Google Scholar]
- 51. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D, Kirklin JK, Pagani FD, Cowger JA. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 2021; 111: 778–792 Epub 2021 Jan 16. PMID: 33465365. [DOI] [PubMed] [Google Scholar]
- 52. van Vugt J, Coebergh van den Braak R, Schippers H, Veen KM, Levolger S, de Bruin R, Koek M, Niessen WJ, IJzermans J, Willemsen F. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr (Edinburgh, Scotland) 2018; 37: 1707–1714. [DOI] [PubMed] [Google Scholar]


