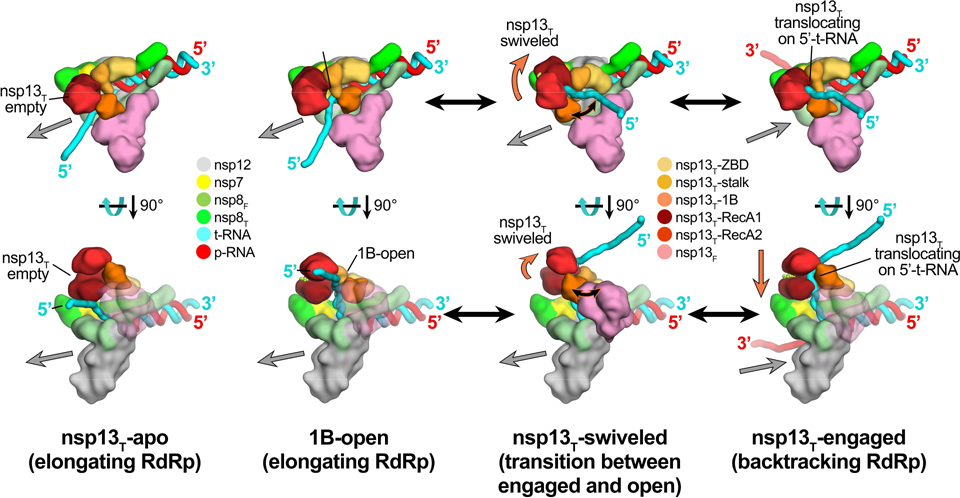
Fig. 6. Schematic model for RTC elongation (1B-open) vs. backtracking (nsp13T-engaged) states.
Top views (top row) and side views (bottom row) of each structural class.
Nsp13T-apo (17%): The nsp13T RecA domains are open, consistent with the absence of nucleotide. Nsp13T is therefore not engaged with the downstream 5’-t-RNA and the RdRp can freely translocate on the t-RNA with concurrent elongation of the p-RNA (gray arrow pointing downstream).
1B-open (33%): The nsp13T 1B domain is rotated open and sterically trapped by the presence of nsp13F. The nsp13T is therefore unable to engage with the downstream 5’-t-RNA and is inactive. The RdRp is able to elongate freely in the downstream direction.
Nsp13T-swiveled (17%): The rotation of the nsp13T protomer away from nsp13F provides space for the nsp13T 1B domain to open and/or close. We therefore propose that nsp13T-swiveled represents a transition state between the 1B-open (elongating) and nsp13T-engaged (backtracking) states.
Nsp13T-engaged (33%): The nsp13T 1B and RecA domains are clamped onto the downstream 5’-t-RNA. In this state, nsp13T can translocate on the t-RNA in the 5’−3’ direction (shown by the orange arrow). This counteracts RdRp elongation and causes backtracking (backward motion of the RdRp on the RNA, shown by the gray arrow pointing upstream).
Also see Extended Data Fig. 8 and Supplementary Videos 1 and 2.