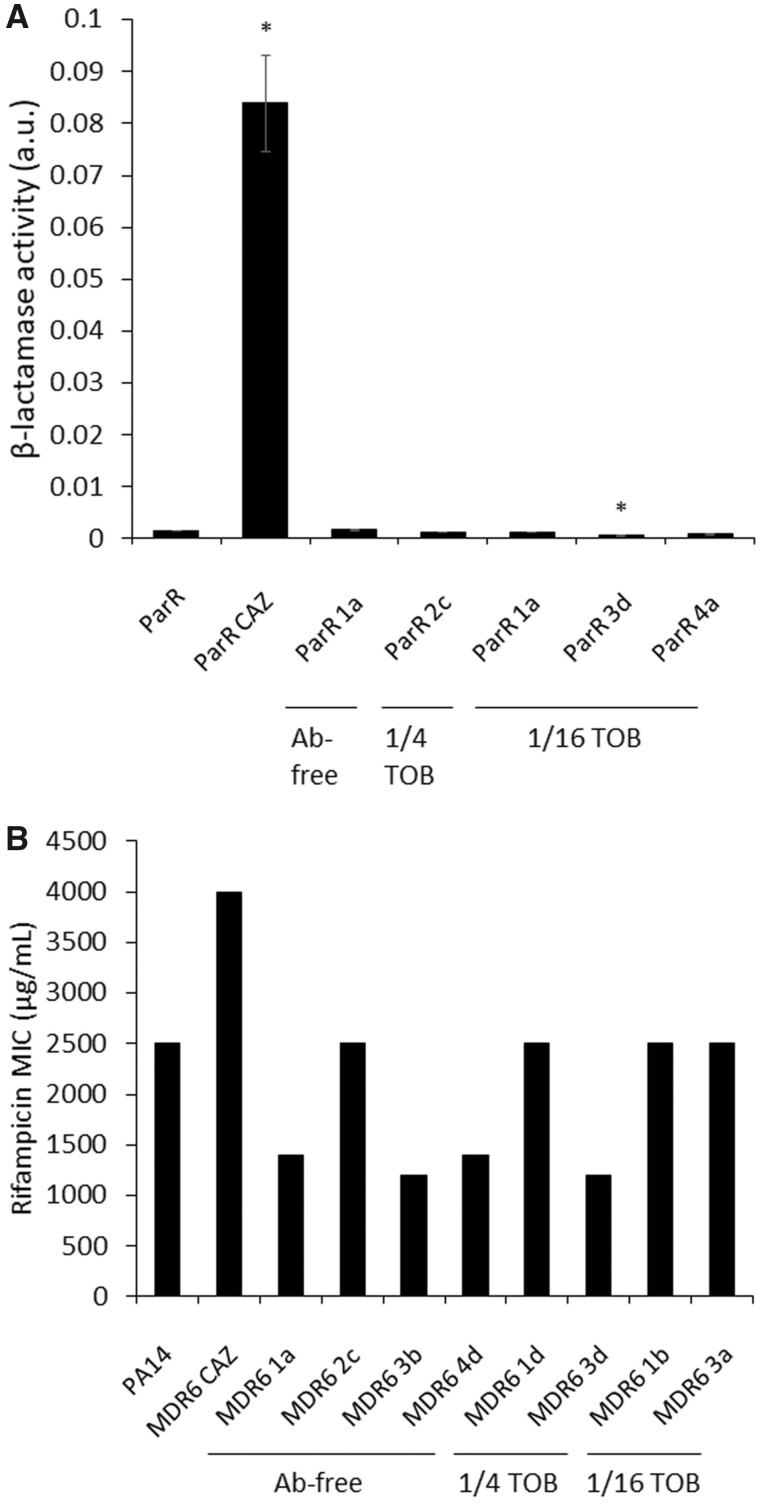
Fig. 6.
Reversion of phenotypes due to intragenic secondary mutations in ampR and rpoB. (A) Figure shows β-lactamase activity of protein extracts from ParR, ParR-CAZ, and Par-CAZ-derived clones: ParR 1a, isolated from a population evolved in antibiotic-free medium, ParR 2c isolated from a population evolved in 1/4 of tobramycin, or ParR 1a, 3d, and 4a isolated from populations evolved in 1/16 of tobramycin MIC sublethal concentration. β-Lactamase activity was increased up to 55-fold in ParR-CAZ mutant respect to its parental strain, whereas a reversion of this phenotype was achieved after 56 days of ALE in antibiotic-free and tobramycin sublethal environments and the acquisition of secondary mutations in ampR. Even a significantly reduced β-lactamase activity respect to the original ParR parental strain was measured for one of them. Error bars indicate standard deviations of the results from three independent biological replicates. Statistically significant differences regarding ParR were calculated with t-test for paired samples assuming equal variances: *P < 0.05. (B) Rifampicin MIC (μg/ml) of PA14 wild-type strain, MDR6-CAZ parental strain, MDR6-CAZ-derived clones: MDR6 1b, 2c, 3b, and 4d isolated from populations evolved in antibiotic-free medium, clones MDR6 1d and 3d isolated from populations evolved in 1/4 of tobramycin MIC sublethal concentration, and clones MDR6 1b and 3a isolated from populations evolved in 1/16 of tobramycin MIC sublethal concentration are represented. As shown, MDR6-CAZ parental strain has an increased rifampicin resistance respect to PA14 wild-type strain, whereas a reversion of this phenotype was achieved after 56 days of ALE in antibiotic-free and tobramycin sublethal environments and the acquisition of secondary mutations in rpoB.