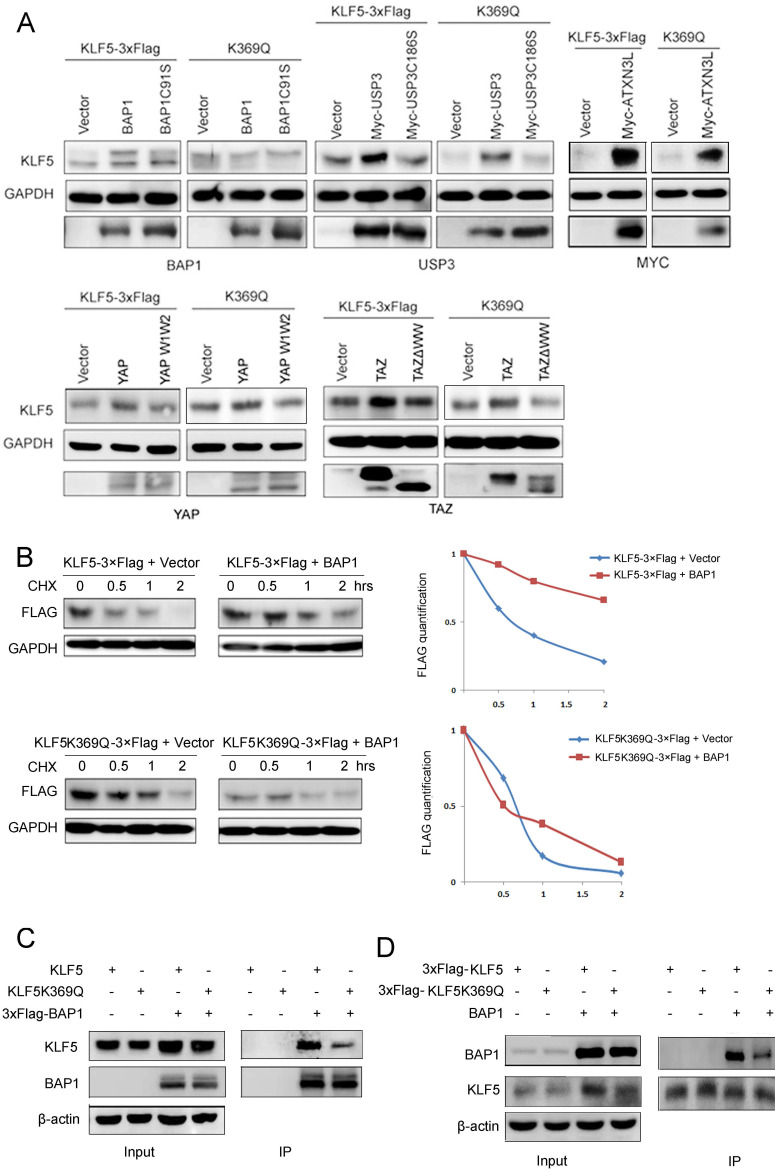
Figure 4.
KLF5 acetylation at K369 suppressed its interaction with BAP1. A, BAP1 did not stabilize KLF5-K369Q protein. KLF5 and KLF5-K369Q were co-transfected with known KLF5 protein stabilizers (BAP1, USP3, ATXN3L, YAP, and TAZ) in HEK293T cells. Protein expression levels were detected by western blotting. B, BAP1 extended the protein half-life of WT KLF5, but not KLF5-K369Q. KLF5-3×Flag and KLF5-K369Q-3×Flag were co-transfected with empty vector and BAP1 in HEK293T cells. The cells were treated with CHX (50 μg/mL) for 0.5, 1, and 2 h. The quantitative results were plotted on the right. C, K369Q mutation decreased the protein interaction between KLF5 and BAP1. KLF5, KLF5-K369Q, and 3×Flag-BAP1 were co-expressed in HEK293T cells. 3×Flag-BAP1 was immunoprecipitated with anti-Flag-M2 beads. Protein expression levels were detected by Western blotting. D, K369Q mutation decreased the protein interaction between KLF5 and BAP1. KLF5-3×Flag, KLF5-K369Q-3×Flag, and BAP1 were co-expressed in HEK293T cells. KLF5-3×Flag and KLF5-K369Q-3×Flag proteins were immunoprecipitated with anti-Flag-M2 beads. Protein expression levels were detected by Western blotting.