Abstract
Background
Maternal lifestyle is discussed as a modifiable determinant in the prevention of preterm birth. However, previous research on associations between individual lifestyle factors and preterm birth risk is inconclusive. In this secondary analysis, we investigated the associations between several modifiable antenatal lifestyle factors and the odds of preterm birth.
Methods
This secondary cohort analysis used data from the cluster-randomised controlled “healthy living in pregnancy” (GeliS) trial. Data were collected from early pregnancy to birth with maternity records, validated questionnaires and birth protocols. Women with complete datasets for all covariates were eligible for analysis. Multivariate logistic regression models, adjusted for recognised risk factors, were fitted to determine whether dietary quality, assessed with a healthy eating index (HEI), physical activity (PA) levels and antenatal anxiety/distress influenced the odds of preterm birth. Moreover, the combined association between pre-pregnancy body mass index (BMI) and HEI on the odds of preterm birth was explored. The independent associations of individual dietary components and types of PA on prematurity were assessed by adjusted logistic regression models.
Results
Overall, 1738 women were included in the analysis. A low HEI significantly increased the odds of preterm birth (OR 1.54 (CI 1.04 – 2.30), p = 0.033), while no associations with either low PA levels or antenatal anxiety/distress were observed. BMI significantly interacted with HEI on the association with prematurity (p = 0.036). Energy % from protein and the intake of average portions of vegetables and cereals were significantly negatively associated with the odds of preterm birth. There was no significant evidence of an association between different types of PA and prematurity.
Conclusions
This cohort analysis revealed that low dietary quality in early pregnancy may increase the chance of giving birth prematurely, while healthier dietary choices may help to prevent preterm birth. More research on pre- and early pregnancy modifiable lifestyle factors is warranted.
Trial registration
This trial is registered with the Clinical Trial Registry ClinicalTrials.gov (NCT01958307). Registration date 09 October 2013, retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-022-04513-5.
Keywords: Preterm birth, Pregnancy, Healthy eating index, Antenatal lifestyle, Risk factors, Diet, Physical activity, Mental health
Background
Preterm birth is the leading cause of death in children under the age of five years [1]. Globally, around 15 million infants are born too soon [2]. The World Health Organization (WHO) defines prematurity as birth before 37 completed weeks of gestation [3]. Although the highest prevalence rates are observed in low- and middle-income countries, where adequate prenatal care is often lacking [2, 4], preterm birth remains a global health issue, with increasing rates in 62 out of 65 countries with reliable data [4]. In Germany, 8 – 9% of babies are born prematurely, which is one of the highest rates observed in Europe [5]. Preterm birth can affect the short-term health status of the newborn, including severe and life-threatening diseases such as necrotising enterocolitis, retinopathy, failure to thrive, metabolic disturbances and sepsis [3, 6]. It is also thought to influence the long-term health outcomes of both the infants and their families, with severe implications regarding their overall quality of life [3, 6]. In order to develop effective and efficacious prevention strategies there is an urgent need to elucidate the aetiology of preterm birth.
The causes of preterm birth are multifactorial and may be sociodemographic, biological, clinical, social-behavioural and environmental in nature [7, 8]. Although several risk factors have been discussed, including a history of preterm birth, infections, smoking during pregnancy, young and advanced age, extremes in body mass index (BMI) and socioeconomic disadvantages [7, 8], approximately two-thirds of preterm births in high-income countries occur for unknown reasons [9]. Thus, identification of further and, in particular, modifiable risk factors is urgently warranted. This has also been emphasised by global healthcare and parent organisations, such as the WHO, the Preterm Birth International Collaborative (PREBIC), and the European Foundation for the Care of Newborn Infants (EFCNI), who highlight the importance of maternal antenatal health and lifestyle parameters concerning preterm birth prevention [8, 10–12].
Previous studies have investigated potential associations between individual maternal lifestyle factors and the risk of preterm birth, including diet [13–22], physical activity (PA) [23, 24] and mental health [25–28]. Yet, findings have been inconclusive, and heterogeneity between studies made comparisons difficult. While some studies found that healthier dietary patterns [15, 20, 22], higher levels of PA [23, 24] and reduced levels of perceived stress [28, 29] lowered the risk of preterm birth, others could not confirm these associations [13, 17, 26, 30]. Given that the aetiology of preterm birth is multifactorial, factors may synergistically or antagonistically influence each other. It is therefore important to simultaneously consider the associations of potential modifiable and non-modifiable predictors on the odds of preterm birth. Moreover, most of the previous studies focused on factors in the second or third trimester [31, 32]. However, manifestation of beneficial lifestyle modifications is a matter of time, thus, identifying risk factors in pre- or early pregnancy may be of higher clinical relevance in terms of preterm birth prevention.
The objective of this cohort analysis was therefore to simultaneously investigate the associations between several pre- and early lifestyle risk factors, including dietary quality, PA levels and anxiety/distress, as well as putative socioeconomic and health risk factors on the odds of preterm birth. We additionally examined the potential influence of certain food groups and different types of PA on the odds of preterm birth.
Methods
Study design and participants
The cluster-randomised controlled “Gesund leben in der Schwangerschaft” (“healthy living in pregnancy”) (GeliS) trial was embedded in routine prenatal care and aimed at reducing the proportion of women with excessive gestational weight gain (GWG), as defined by the Institute of Medicine (IOM) [33]. Results on the primary and secondary endpoints have been reported elsewhere [34–40]. The study was conducted in accordance with the declaration of Helsinki and was approved by the ethics committee of the Technical University of Munich. A comprehensive description of the rationale, study design and methods is provided in the study protocol [33] and the trial register entry (ClinicalTrials.gov, NCT01958307).
In brief, participating midwifery and gynaecological practices from five regions in Bavaria, Germany, recruited pregnant women between 2013 and 2015. Women were eligible for participation if they met the following inclusion criteria: (1) pre-pregnancy BMI ≥ 18.5 kg/m2 and ≤ 40.0 kg/m2, (2) singleton pregnancy, (3) aged between 18 and 43 years, (4) ≤ 12 weeks of gestation, (5) sufficient German language skills, and (6) provision of written informed consent. Women were not eligible if they had multiple or complicated pregnancies or chronic diseases.
Women in the control group received standard prenatal care and a leaflet containing general information on healthy lifestyle and breastfeeding. Participants in the intervention group were offered three counselling sessions during pregnancy and one in the postpartum period alongside their routine care visits. Lifestyle counselling was given by previously trained midwives, gynaecologists or medical personnel and comprised personalised advice on recommended GWG, healthy diet, PA and breastfeeding [33].
Data collection and study outcomes
Preterm and full-term birth
Data on gestational age at birth were derived from birth records. Preterm birth was defined as a live birth before 37 completed weeks of gestation, including both spontaneous and iatrogenic (induced labor or planned caesarean section) preterm birth. Preterm birth was further categorised into extremely, very, and moderate-to-late preterm born, referring to a gestational age of < 28 weeks, 28 – < 32 weeks, and 32 – < 37 weeks, respectively [3]. Infants born at 37 completed weeks of gestation or after were categorised as full-term birth.
Sociodemographic and clinical data
Maternal sociodemographic data were obtained by a screening questionnaire before the 12th week of gestation. Educational level was classified as low when women graduated from general secondary school or lower.
Maternal pre-pregnancy weight was likewise collected via the screening questionnaire. The self-reported weight was used to categorise women into pre-pregnancy BMI classes (normal weight (BMI 18.5 – 24.9 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), and obesity (BMI 30.0 – 40.0 kg/m2)). Maternal weight during pregnancy was measured at each antenatal visit and recorded in maternity records. Early pregnancy GWG was calculated by subtracting the first measured weight in pregnancy (≤ 12 weeks of gestation) from the weight measured at the 16th to 20th week of gestation. Early GWG was further categorised into inadequate, adequate and excessive GWG according to the weekly GWG recommendations from the IOM [41]. It was assumed that women gain around 1 – 2 kg in the first trimester. Inadequate and excessive GWG were defined based on the lower (1 kg) and higher (2 kg) end of the range, respectively.
Between the 24th and the 28th week of gestation, women underwent a standardised 75 g oral glucose tolerance test (oGTT). According to national and international guidelines, women were diagnosed with gestational diabetes mellitus (GDM) if one or more of the following cut-off values was equalled or exceeded: Fasting plasma glucose: 92 mg/dL (5.1 mmol/L), 1 h: 180 mg/dL (10.0 mmol/L), and 2 h: 153 mg/dL (8.5 mmol/L) [42, 43].
Lifestyle factors
Women were asked to fill out a set of validated questionnaires in early pregnancy (≤ 12 weeks of gestation), covering pre- and early pregnancy lifestyle factors, including dietary and PA behaviour, smoking status, and mental health. Mothers who smoked during early pregnancy were classified as current smokers. Data on dietary intake over the last month were collected using the validated, and slightly modified, food frequency questionnaire (FFQ) originally developed by the Robert Koch Institute in Berlin, Germany, and applied in the German Health Interview and Examination Survey for Adults (DEGS) study [44]. This questionnaire enquired the consumption frequency and portion size of 54 different food items. Based on the collected dietary information, energy, macronutrient and fibre intake were estimated. Over- and underreporting of dietary behaviour was defined as previously reported [38], and women over- or underreporting dietary intake were excluded from analysis. Based on the FFQ, a healthy eating index (HEI) was calculated to estimate the dietary quality in accordance with the German national recommendations [45]. The score comprises values between 0 and 100. A score of 0 indicates the lowest and a score of 100 indicates the highest dietary quality. The median of women’s total HEI was used as a cut-off to categorise them as having a low or high HEI.
Data on PA behaviour were obtained using the Pregnancy Physical Activity Questionnaire (PPAQ), which has been slightly adapted to fit German habits [46]. The PPAQ comprises 32 questions related to the type and intensity of women’s PA behaviour during the last month. Based on the evaluation sheet of the PPAQ, time and intensity spent for each of the 32 activities was summed up to obtain the average weekly energy expenditure in metabolic equivalent of task (MET)-h/week [46]. Overreporting was defined as described elsewhere [35] and questionnaires of women overreporting their PA behaviour were excluded from analysis. MET-h/week of light intensities and above were summed up to total physical activity of light intensity and above (TALIA). The median of TALIA was used as cut-off to group women into high (above the median) or low (below the median) PA behaviour. Types of PA behaviour were derived from the PPAQ and included household, occupational, transportation, sports activity and inactivity.
Data on maternal mental health were collected using the German version of the Patient Health Questionnaire-4 (PHQ-4). A score of ≥ 3 on a scale of maximum 12 points indicates symptoms of anxiety and depression [47].
Statistical analysis
Women with available information on preterm birth and complete data for all of the covariates listed above were included in the analysis. Participants who dropped out during pregnancy, as well as women with missing or invalid lifestyle data due to over- or underreporting, were excluded from analysis (see Fig. 1 for reasons of drop out). As the primary focus of this analysis was to identify antenatal predictors of preterm birth, and as there was no significant difference between intervention and control group in the incidence of preterm birth (Additional file 1: Table S1), data of both groups were pooled to form one cohort. In all analyses, group assignment was included as an adjustment factor to prevent potential confounding.
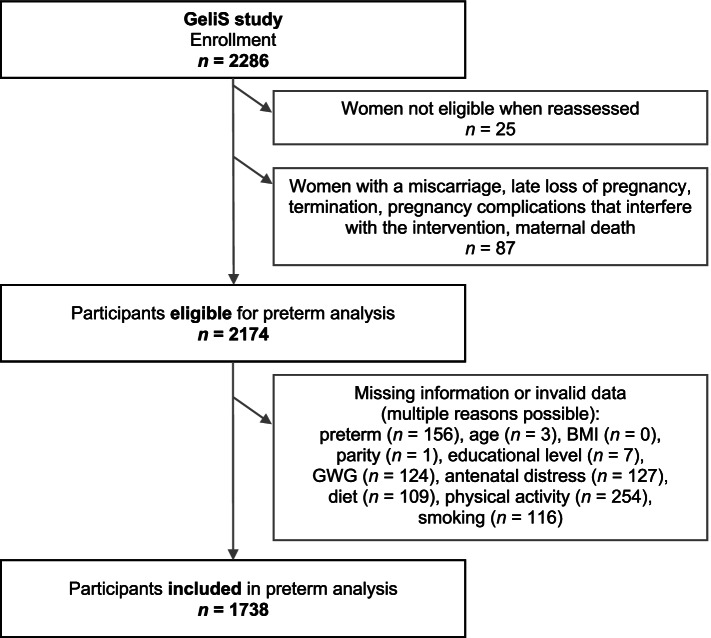
Fig. 1.
Flowchart of participants enrolled in the GeliS trial and included in preterm analysis. BMI body mass index, GWG gestational weight gain
Baseline characteristics were stratified according to preterm birth status and are presented as means and standard deviations (SD) or frequencies and proportions. Differences between the groups were assessed by Chi-squared test for categorical and by Kruskal-Wallis test for continuous variables.
Multivariate logistic regression models were used to examine associations between potential predictors and the odds of preterm birth. Odds ratios (OR) with 95% confidence intervals (CI) were calculated. Four models with different sets of categorical and equivalent linear covariates were fitted. Model 1 included group assignment, maternal age, and pre-pregnancy BMI as covariates. Model 2 additionally assessed the influence of early inadequate or excessive GWG and nulliparity on the odds of preterm birth. Model 3 further comprised low education and smoking status. For the fully adjusted Model 4, low HEI, low PA and antenatal anxiety and distress were added. We further explored the potential combined association of HEI and BMI on the odds of preterm birth by including their interaction term into the fully adjusted categorical Model 4. Further logistic regression models were fitted to examine the influence of individual dietary and PA variables on the odds of preterm birth, adjusting for group assignment, maternal age, parity, and pre-pregnancy BMI.
Exploratory subgroup analyses of the multivariate logistic regression models were performed to assess the associations between the aforementioned covariates and the odds of spontaneous and iatrogenic preterm birth.
All analyses were performed using SPSS software (IBM SPSS Statistics for Windows, version 26.0, IBM Corp, Armonk, New York). A p value of < 0.05 was considered statistically significant. Due to the exploratory nature of this analysis, no adjustment for multiple comparisons was perfomed.
Results
Study sample and participant characteristics
Among 2286 enrolled women in the GeliS study, 2174 women were potentially eligible for the current analysis (Fig. 1). After exclusion of women with missing or implausible data for any of the covariates, the final analytical sample amounted to 1738 women. The characteristics of the original eligible and the finally included sample were comparable (Additional file 1: Table S2). The preterm birth rate did not differ between intervention and control groups (Additional file 1: Table S1).
Table 1 summarises maternal and infant characteristics of the included study population, stratified by preterm birth status. Overall, mothers were on average 30.4 ± 4.4 years old and had a mean pre-pregnancy BMI of 24.4 ± 4.5 kg/m2. Among the 1738 women, 114 (6.6%) had a preterm birth, of whom 100 (5.8%) were classified as moderate to late preterm, 12 (0.7%) as very preterm, and 2 (0.1%) as extremely preterm. Of the 112 women who gave birth to a preterm infant with additional information on the type of birth, 77 (68.8%) had a spontaneous and 35 (31.3%) had an iatrogenic preterm birth (Additional file 1: Table S3). Infants born preterm had lower birthweights as compared to infants born full-term (2370 ± 622 g vs. 3405 ± 436 g, p < 0.001) (Table 1). Mothers who gave birth to a preterm infant were more likely to be nulliparous compared to mothers who gave birth to a full-term infant (66.7% vs. 57.5%, p = 0.055). The proportion of mothers with a low HEI score was higher among those with a preterm birth, as compared to those with a full-term birth (59.6% vs. 49.3%, p = 0.033). All other sociodemographic, health and lifestyle factors were comparable between groups (Table 1).
Table 1.
Characteristics of study participants with full-term vs. preterm birth
|
Full-term (n = 1624, 93.4%) |
Preterm (n = 114, 6.6%) |
Total (n = 1738) |
p valuea | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Group allocationb | ||||
| Control group | 792/1624 (48.8%) | 50/114 (43.9%) | 842/1738 (48.4%) | 0.311 |
| Intervention group | 832/1624 (51.2%) | 64/114 (56.1%) | 896/1738 (51.6%) | |
| Pre-pregnancy age (years)c | 30.3 ± 4.4 | 31.0 ± 4.4 | 30.4 ± 4.4 | 0.139 |
| Pre-pregnancy weight (kg) | 68.2 ± 13.5 | 67.9 ± 13.2 | 68.2 ± 13.4 | 0.906 |
| Pre-pregnancy BMI (kg/m2) | 24.3 ± 4.5 | 24.6 ± 4.5 | 24.4 ± 4.5 | 0.479 |
| Pre-pregnancy BMI category (n (%)) | ||||
| BMI 18.5 – 24.9 kg/m2 | 1069/1624 (65.8%) | 68/114 (59.6%) | 1137/1738 (65.4%) | 0.332 |
| BMI 25.0 – 29.9 kg/m2 | 362/1624 (22.3%) | 32/114 (28.1%) | 394/1738 (22.7%) | |
| BMI 30.0 – 40.0 kg/m2 | 193/1624 (11.9%) | 14/114 (12.3%) | 207/1738 (11.9%) | |
| Early GWG (kg)d | 2.8 ± 2.4 | 2.6 ± 2.2 | 2.8 ± 2.4 | 0.381 |
| Early GWG category (n (%))e | ||||
| Inadequate | 432/1624 (26.6%) | 34/114 (29.8%) | 466/1738 (26.8%) | 0.754 |
| Adequate | 196/1624 (12.1%) | 13/114 (11.4%) | 209/1738 (12.0%) | |
| Excessive | 996/1624 (61.3%) | 67/114 (58.8%) | 1063/1738 (61.2%) | |
| GDM (n (%))f | 166/1566 (10.6%) | 14/109 (12.8%) | 180/1675 (10.7%) | 0.465 |
| Educational level (n (%)) | ||||
| General secondary schoolg | 227/1624 (14.0%) | 18/114 (15.8%) | 245/1738 (14.1%) | 0.835 |
| Vocational secondary school | 704/1624 (43.3%) | 47/114 (41.2%) | 751/1738 (43.2%) | |
| Academic high school | 693/1624 (42.7%) | 49/114 (43.0%) | 742/1738 (42.7%) | |
| Country of birth (n (%)) | ||||
| Germany | 1459/1622 (90.0%) | 97/114 (85.1%) | 1556/1736 (89.6%) | 0.100 |
| Other | 163/1622 (10.0%) | 17/114 (14.9%) | 180/1736 (10.4%) | |
| Native language (n (%)) | ||||
| German | 1537/1622 (94.8%) | 109/114 (95.6%) | 1646/1736 (94.8%) | 0.691 |
| Other | 85/1622 (5.2%) | 5/114 (4.4%) | 90/1736 (5.2%) | |
| Nulliparous (n (%)) | 934/1624 (57.5%) | 76/114 (66.7%) | 1010/1738 (58.1%) | 0.055 |
| Living with a partner (n (%)) | 1564/1619 (96.6%) | 111/114 (97.4%) | 1675/1733 (96.7%) | 0.660 |
| Married (n (%)) | 1077/1618 (66.6%) | 70/114 (61,4%) | 1147/1732 (66.2%) | 0.260 |
| Full-time employed (n (%)) | 857/1612 (53.2%) | 69/113 (61.1%) | 926/1725 (53.7%) | 0.104 |
| Current smoker (n (%)) | 81/1624 (5.0%) | 4/114 (3.5%) | 85/1738 (4.9%) | 0.479 |
| Low HEI (n (%))h | 801/1624 (49.3%) | 68/114 (59.6%) | 869/1738 (50.0%) | 0.033 |
| Low PA (n (%))i | 807/1624 (49.7%) | 62/114 (54.4%) | 869/1738 (50.0%) | 0.333 |
| Antenatal distress (n (%))j | 682/1624 (42.0%) | 48/114 (42.1%) | 730/1738 (42.0%) | 0.982 |
| Low well-being (n (%))k | 589/1609 (36.6%) | 38/110 (34.5%) | 627/1719 (36.5%) | 0.664 |
| Infant characteristics | ||||
| Infant sex (n (%)) | ||||
| Male | 825/1608 (51.3%) | 62/110 (56.4%) | 887/1718 (51.6%) | 0.304 |
| Female | 783/1608 (48.7%) | 48/110 (43.6%) | 831/1718 (48.4%) | |
| Birthweight (g) | 3405 ± 436 | 2370 ± 622 | 3337 ± 518 | < 0.001 |
BMI body mass index, GDM gestational diabetes mellitus, GWG gestational weight gain, HEI Healthy Eating Index, IOM Institute of Medicine, oGTT oral glucose tolerance test, PA physical activity, PHQ-4 Patient Health Questionnaire-4, SD standard deviation, TALIA total physical activity of light intensity and above, WHO-5 World Health Organization Well-Being Index 5
ap value for differences between women with full-term and preterm birth, tested with χ2 test for categorical variables and Kruskal-Wallis test for continuous variables
bFrequency (percent) (all such values)
cMean ± SD (all such values)
dGWG until the 16th – 20th week of gestation
eDefined according to the criteria of the IOM
fAssessed by an 75 g oGTT in the 24th – 28th week of gestation
gGeneral secondary school, which is completed through year 9
hHEI below the median of the analysed population
iTALIA below the median of the analysed population
jPHQ-4 score of ≥ 3 points
kWHO-5 score of < 50%
Associations between pre- and early pregnancy sociodemographic, health and lifestyle factors and the odds of preterm birth
Multivariate logistic regression analyses revealed significant associations between both nulliparity and advanced age (36 – 43 years) and the odds of preterm birth across Models 2 to 4 (Table 2). In the fully adjusted Model 4, low HEI was significantly positively associated with the odds of preterm birth (OR 1.54 (CI 1.04 – 2.30), p = 0.033). There was no significant evidence of an association between pre-pregnancy BMI, excessive or inadequate GWG, low PA, antenatal anxiety/distress, smoking or low education and the odds of preterm birth (Table 2).
Table 2.
Associations between sociodemographic, health and lifestyle factors and the odds of preterm birth
| Covariate | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Group assignment | 1.20 (0.82 – 1.76) | 1.14 (0.78 – 1.68) | 1.15 (0.78 – 1.69) | 1.18 (0.80 – 1.73) |
| BMI categorya | ||||
| BMI 25.0 – 29.9 kg/m2 | 1.39 (0.89 – 2.15) | 1.43 (0.92 – 2.22) | 1.41 (0.90 – 2.20) | 1.37 (0.88 – 2.14) |
| BMI 30.0 – 40.0 kg/m2 | 1.14 (0.63 – 2.08) | 1.10 (0.60 – 2.02) | 1.09 (0.59 – 1.99) | 1.04 (0.56 – 1.91) |
| Ageb | ||||
| 26 – 35 years | 1.52 (0.78 – 2.98) | 1.69 (0.86 – 3.32) | 1.70 (0.86 – 3.36) | 1.82 (0.92 – 3.63) |
| 36 – 43 years | 2.06 (0.94 – 4.55) | 2.52 (1.12 – 5.65)+ | 2.53 (1.13 – 5.67)+ | 2.70 (1.20 – 6.10)+ |
| Nulliparity | 1.63 (1.08 – 2.45)+ | 1.64 (1.08 – 2.47)+ | 1.58 (1.02 – 2.42)+ | |
| Early GWGc | ||||
| Excessive | 0.99 (0.53 – 1.84) | 1.00 (0.54 – 1.86) | 0.98 (0.53 – 1.83) | |
| Inadequate | 1.23 (0.63 – 2.41) | 1.25 (0.64 – 2.44) | 1.18 (0.60 – 2.32) | |
| Smokingd | 0.70 (0.25 – 1.97) | 0.64 (0.23 – 1.81) | ||
| Low educatione | 1.25 (0.73 – 2.14) | 1.18 (0.68 – 2.03) | ||
| Low HEIf | 1.54 (1.04 – 2.30)+ | |||
| Low PAg | 1.04 (0.69 – 1.55) | |||
|
Antenatal anxiety/ distressh |
1.01 (0.69 – 1.50) | |||
BMI body mass index, GWG gestational weight gain, HEI Healthy Eating Index, IOM Institute of Medicine, PA physical activity, PHQ-4 Patient Health Questionnaire-4, TALIA total physical activity of light intensity and above
+ p < 0.05
aBMI 18.5 – 24.9 kg/m2 was used as reference
bAge 18 – 25 years was used as reference
cGWG until the 16th – 20th week of gestation, defined according to the criteria of the IOM, adequate GWG was used as reference
dCurrent smoker
eGeneral secondary school or lower
fHEI below the median of the analysed population
gTALIA below the median of the analysed population
hPHQ-4 score of ≥ 3 points
In an exploratory analysis a significant interactive association between pre-pregnancy BMI and HEI on the odds of preterm birth was observed (p = 0.036, data not shown).
Multivariate analyses were also performed with the inclusion of the same predictor variables, noted above, as continuous variables. Age was significantly positively associated with the odds of preterm birth across Models 2 to 4 (Model 4: OR 1.05 (1.00 – 1.10), p = 0.037) (Additional file 1: Table S4). None of the other factors were significantly linked to the odds of preterm birth in the linear models.
Overall, the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were lower across categorical as compared to linear models (data not shown). Lower information criteria indicate a better fit of the model [48].
Subgroup analyses exploring possible associations of the included covariates with the type of preterm birth revealed advanced age to be significantly positively associated with the odds of iatrogenic preterm birth across categorical Models 2 to 4 (Model 4: OR 5.99 (1.20 – 29.79), p = 0.029) and across linear Models 1 to 4 (Model 4: OR 1.12 (1.04 – 1.22), p = 0.004) (Additional File 1: Table S5 and Table S6). Nulliparity was significantly positively associated with the odds of spontaneous preterm birth across categorical Models 2 to 3 (Model 3: OR 1.69 (1.02 – 2.80), p = 0.043) (Additional file 1: Table S7 and Table S8).
Associations between specific dietary and physical activity variables and the odds of preterm birth
Table 3 depicts results from regression analyses investigating whether the intake of certain macronutrients, food groups and different types of PA are associated with the odds of preterm birth. There was no evidence of an association between overall energy intake and the odds of preterm birth, while energy % (E%) from certain macronutrients and food groups was associated with prematurity. The odds of preterm birth were significantly decreased by 53% per 10 E% derived from protein (p = 0.030). E% from both carbohydrates in general and from saccharose tended to increase the odds of preterm birth, without evidence of a robust association (p = 0.071 and p = 0.062, respectively).
Table 3.
Associations between specific dietary and physical activity variables and the odds of preterm birth
| n | OR (CI) | p valuea | |
|---|---|---|---|
| Energy and macronutrient intake | |||
| Energy intake (per 100 kcal/day) | 1584 | 1.00 (0.97 – 1.04) | 0.940 |
| E% fat (per 10 E%/day) | 1584 | 0.80 (0.57 – 1.11) | 0.175 |
| E% saturated fat (per 10 E%/day) | 1584 | 0.74 (0.40 – 1.38) | 0.345 |
| E% protein (per 10 E%/day) | 1584 | 0.47 (0.24 – 0.93) | 0.030 |
| E% carbohydrates (per 10 E%/day) | 1584 | 1.27 (0.98 – 1.64) | 0.071 |
| Saccharose (per 10 g/day) | 1584 | 1.06 (0.98 – 1.12) | 0.062 |
| Fibre (per 10 g/day) | 1584 | 0.93 (0.76 – 1.15) | 0.511 |
| Alcohol (per g/day) | 1584 | 1.06 (0.99 – 1.14) | 0.097 |
| Caffeine (100 mg/day) | 1582 | 1.05 (0.82 – 1.33) | 0.710 |
| Food intake | |||
| Soft drinks (200 ml/day) | 1737 | 1.01 (0.95 – 1.07) | 0.843 |
| Sweets and snacks (50 g/day) | 1738 | 0.95 (0.79 – 1.15) | 0.628 |
| Fast food (250 g/day) | 1737 | 1.04 (0.27 – 4.00) | 0.961 |
| Meat and meat products (150 g/day) | 1737 | 0.79 (0.45 – 1.38) | 0.408 |
| Fish (90 g/day) | 1737 | 0.61 (0.14 – 2.62) | 0.508 |
| Dairy products (200 g/day) | 1738 | 0.86 (0.73 – 1.02) | 0.078 |
| Cheese (30 g/day) | 1734 | 0.82 (0.64 – 1.05) | 0.108 |
| Eggs (60 g/day) | 1727 | 0.49 (0.19 – 1.26) | 0.138 |
| Cereals (50 g/day) | 1738 | 0.71 (0.59 – 0.85) | < 0.001 |
| Nuts (25 g/day) | 1734 | 0.54 (0.16 – 1.80) | 0.313 |
| Fruits (150 g/day) | 1737 | 1.05 (0.97 – 1.14) | 0.234 |
| Vegetables (150 g/day) | 1736 | 0.75 (0.59 – 0.96) | 0.023 |
| Vegetarian | 1727 | 0.66 (0.26 – 1.65) | 0.372 |
| Physical activityb | |||
| Total PA | 1738 | 1.02 (0.70 – 1.49) | 0.924 |
| Household PA | 1738 | 0.98 (0.94 – 1.02) | 0.333 |
| Occupational PA | 1330 | 1.00 (0.99 – 1.00) | 0.694 |
| Sports | 1738 | 0.92 (0.74 – 1.14) | 0.447 |
| Transportation PA | 1738 | 0.94 (0.80 – 1.11) | 0.462 |
| Inactivity | 1728 | 1.07 (0.94 – 1.23) | 0.287 |
CI confidence interval, E% energy percent, MET metabolic equivalent of task, OR odds ratio, PA physical activity
aAdjusted for maternal pre-pregnancy BMI, age, parity and group assignment
bEffect sizes are calculated per 10 MET-h/week
Among food groups, vegetable intake and cereal intake, per average portion, lowered the odds of preterm birth by 25% (p = 0.023) and by 29% (p < 0.001), respectively. Consumption of dairy products yielded a similar trend (p = 0.078). No further food groups were significantly associated with the odds of preterm birth (Table 3).
There was no evidence of associations for total PA or any of the different types of PA, including household, occupational, sports, transportation or inactivity with the odds of giving birth to a preterm infant (Table 3).
Discussion
This secondary analysis of pooled data from the GeliS cohort investigated the simultaneous associations between several modifiable and non-modifiable pre- and early pregnancy, sociodemographic, health and lifestyle factors and the risk of preterm birth. The findings suggest that women with a low HEI in early pregnancy have a higher risk of giving birth to a preterm infant. An exploratory analysis further elucidated that there was a significant combined association between low dietary quality and higher pre-pregnancy BMI on the risk of preterm birth. More specific associations between selected dietary factors and preterm birth risk revealed that E% from protein and the intake of vegetables and cereals reduced the odds. None of the other modifiable risk factors, including low PA and anxiety or distress in pre- and early pregnancy, were significantly associated with the risk of prematurity.
The causes of prematurity are still largely unknown, which makes the identification of early risk factors highly relevant for clinical practice. Within the last few decades, research has focused on identifying maternal sociodemographic and health characteristics as risk factors for preterm birth. Our data confirmed that advanced age and nulliparity increase the risk for preterm birth, as already noted previously [49]. Subgroup analyses by the type of preterm birth revealed advanced age to increase the risk for iatrogenic and nulliparity to increase the risk for spontaneous preterm birth, which is in line with previous investigations [49–51]. While previous research suggests that extremes in BMI, including underweight and overweight/obesity, and inadequate as well as excessive GWG are risk factors for preterm birth [52–54], we found no evidence of independent associations in our multivariate models. In our analyses, neither smoking nor low education showed an influence on preterm birth risk. Although our findings contradict previous research observing associations with these maternal factors and preterm birth [7, 55], differences might be explained by the relatively high educational level and the rather low rate of smokers in the GeliS cohort [56].
Besides maternal sociodemographic and health characteristics, lifestyle factors are discussed to play a crucial role in preventing preterm birth. So far, published research focused on analysing lifestyle factors independently. However, factors might interact with each other, which emphasizes to explore the combined associations of predictors on preterm birth risk.
Recent observations of lower preterm birth rates during the lockdown phases of the ongoing COVID-19 pandemic suggest that drastic changes to women’s lifestyles may emerge as possible contributory factors [57–61]. Besides changes in dietary and PA habits, one discussed factor is stress, and in particular work related stress, which might have been reduced during the pandemic [57, 61]. In agreement with this hypothesis, a recent cohort analysis reported that perceived stress significantly increased the risk for preterm birth [29]. However, other mental health outcomes, including antenatal depressive symptoms, were not found to be associated with preterm birth risk in this cohort [29], which is in line with our observations. More research is needed to clarify the role of women’s mental health with regard to preterm birth risk.
In addition to maternal mental health, diet and PA have been discussed to influence preterm birth risk. In line with our findings, recent systematic reviews [31, 62, 63] have found that healthier dietary patterns, characterised by a high intake of vegetables, fruits and whole grains, reduce the risk of preterm birth, while unhealthier western-type dietary patterns had the opposite effect [64]. Notably, studies investigating whether certain diets influence the risk for preterm birth were inconsistent. For instance, adherence to a Mediterranean diet has been linked to reduced preterm birth risk in a Danish cohort [20], while no association was observed in a Norwegian cohort [17]. In the Norwegian MoBa cohort, adherence to the new Nordic diet lowered the risk for preterm birth [18]. In two cohorts from Asia and Australia, overall dietary quality assessed by a HEI had no influence on overall preterm birth risk [13, 15], although the Australian study found higher dietary quality to reduce the risk of preterm birth among women with obesity [15]. A similar observation was made by Saunders et al. [19]. In an exploratory analysis, we confirmed pre-pregnancy BMI to significantly interact with HEI on the association with preterm birth risk.
Inconsistencies in findings related to dietary parameters and their contribution to the risk of preterm birth may be attributable to differences in dietary assessment methods, timing of assessment, methodological approaches and heterogeneity in population characteristics, which makes a general comparison between study results difficult.
The potential of PA as another modifiable risk factor for preterm birth risk is not yet fully understood. We did not observe any association between low PA and the odds of preterm birth in the combined model estimate. While some of the previous studies explicitly described leisure time PA/overall PA to reduce preterm birth risk [32, 65], others observed no influence [66, 67]. Our research group previously investigated the impact of different PA intensities on preterm birth risk [68]. Sedentary behaviour tended to increase the risk of preterm birth, as did vigorous PA behaviour in late pregnancy. In line with these observations, other studies have observed a U-shaped trend between PA intensity/amount and preterm birth risk, indicating that light-to-moderate PA seems to be safe and should be aspired to, as its beneficial effects extend beyond risk reduction and enhance a woman’s well-being [23, 69]. Different from our previous investigation on PA intensity [68], the present analysis endeavoured to assess the link between the less investigated types/purposes of PA, including household, transportation, occupation, sports and inactivity and the risk for preterm birth. None of these PA types were associated with prematurity, which is in line with previous literature [32, 65]. These findings lead to the suggestion that not the type of PA per se, but rather PA intensity and potentially also duration seems to confer risk lowering effects on preterm birth.
Limitations
Our findings should be interpreted in light of some limitations. Despite using validated questionnaires to assess maternal lifestyle, self-reported data could have introduced reporting bias. Although we used a validated FFQ, we acknowledge that the calculated E% is only an estimate and may not be completely accurate. Although we adjusted for a variety of non-modifiable and modifiable covariates in our multivariate models, residual confounding by other risk factors, such as ethnicity or a history of preterm birth, cannot be excluded. We are aware that some of the reported OR for the influence of specific food groups are rather small and their clinical relevance has to be interpreted with caution. As the data are taken from a well-educated sample of women from a single federal state, generalising our findings to the broader German population may be limited. The lack of more detailed information on the ethnicity of the study participants is a clear limitation which needs to be acknoweledged. The exclusion of women with complicated or multiple pregnancies, which are known to have an increased risk for preterm birth [7], could explain the lower incidence of preterm births in our cohort compared to the figures for Germany [5]. However, in these women, factors other than lifestyle may play a major role in increasing the risk of preterm birth, making them less relevant to this analysis.
Strengths
This comprehensive analysis has several strengths that merit particular attention. This study adds to the evidence the simultaneous consideration of modifiable and non-modifiable maternal determinants in pre- and early pregnancy on the odds of preterm birth. Moreover, few research groups have examined so far whether pre- and early pregnancy lifestyle factors play a role in increasing the risk for preterm birth. Dietary quality was rated with a HEI. This tool might be advantageous over previous approaches, such as dietary pattern analyses, as it was based on national recommendations. The GeliS study considered women of several BMI classes, which enabled us to identify a combined association between pre-pregnancy BMI and HEI on the odds of prematurity. Our study was conducted within the German routine care system and thus allowed the collection of data from medical records with minimal inconvenience to the participants and, consequently, low drop-out rates.
Conclusions
Preterm birth is a global health issue, for which the causes are still largely unknown. Our findings suggest that a low dietary quality is associated with an increased risk of preterm birth, while a healthier diet may contribute to prevent prematurity. Further research on early modifiable risk factors is needed to develop prevention strategies. Cohort data allowing a pre- and within-pandemic comparison may be additionally valuable for further elucidation.
Supplementary Information
Additional file 1: Table S1 Preterm incidence in intervention and control group. Table S2 Characteristics of eligible and included participants. Table S3 Proportions of spontaneous and iatrogenic preterm births. Table S4 Associations between sociodemographic, health and lifestyle factors and the odds of preterm birth – models including linear covariates. Table S5 Associations between sociodemographic, health and lifestyle factors and the odds of iatrogenic preterm birth – models including categorical covariates. Table S6 Associations between sociodemographic, health and lifestyle factors and the odds of iatrogenic preterm birth – models including linear covariates. Table S7 Associations between sociodemographic, health and lifestyle factors and the odds of spontaneous preterm birth – models including categorical covariates. Table S8 Associations between sociodemographic, health and lifestyle factors and the odds of spontaneous preterm birth – models including linear covariates.
Acknowledgements
We gratefully acknowledge the valuable contribution from our partners and funders, the Competence Centre for Nutrition, the Bavarian State Ministry of Food, Agriculture and Forestry, the Bavarian State Ministry of Health and Care, the AOK Bayern and the Else Kröner-Fresenius foundation, Bad Homburg, Germany. Moreover, we gratefully thank all cooperation partners and the expert advisory board who have been named and acknowledged elsewhere [34]. Finally, we would like to thank our colleagues and former colleagues from the Institute of Nutritional Medicine, Klinikum rechts der Isar, Technical University of Munich, Dr. Kathrin Rauh, Dr. Lynne Stecher, Dr. Julia Kunath, Dr. Christina Holzapfel, Isabel Lück, Annie Naujoks and Lara Donik, and the Competence Centre for Nutrition, Eva Rosenfeld and Luzia Kick for their support, and all participating practices, gynaecologists, medical personnel, midwives, participants and their families for their involvement.
Abbreviations
- WHO
World Health Organization
- BMI
Body Mass Index
- PREBIC
Preterm Birth International Collaborative
- EFCNI
European Foundation for the Care of Newborn Infants
- PA
Physical Activity
- GeliS
Gesund leben in der Schwangerschaft (“healthy living in pregnancy”)
- GWG
Gestational Weight Gain
- IOM
Institute of Medicine
- oGTT
Oral Glucose Tolerance Test
- GDM
Gestational Diabetes Mellitus
- FFQ
Food Frequency Questionnaire
- DEGS
German Health Interview and Examination Survey for Adults
- HEI
Healthy Eating Index
- PPAQ
Pregnancy Physical Activity Questionnaire
- MET
Metabolic Equivalent of Task
- TALIA
Total Physical Activity of Light Intensity and Above
- PHQ-4
Patient Health Questionnaire-4
- SD
Standard Deviation
- OR
Odds Ratio
- CI
Confidence Interval
- AIC
Akaike Information Criterion
- BIC
Bayesian Information Criterion
Authors’ contributions
Hans Hauner, Roxana Raab, Julia Hoffmann, Monika Spies, Kristina Geyer, Dorothy Meyer, and Julia Günther are members of the GeliS study group. Hans Hauner designed the research project, developed the study protocol and established the lifestyle intervention programme. Roxana Raab and Julia Hoffmann developed the research question and were in charge of statistical analyses. Roxana Raab performed the literature search, generated tables and figures and wrote together with Julia Hoffmann the first draft of the manuscript. Monika Spies, Kristina Geyer, Dorothy Meyer, Julia Günther, and Hans Hauner provided scientific support. Monika Spies, Kristina Geyer, Dorothy Meyer, Julia Günther, and Hans Hauner assisted in writing the manuscript. Roxana Raab, Julia Hoffmann, and Hans Hauner had primary responsibility for the final content. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by Else Kröner-Fresenius Foundation, Bad Homburg (Grant number: 5140889), the Else Kröner-Fresenius Centre for Nutritional Medicine at the Technical University of Munich, the Competence Centre for Nutrition (KErn) in Bavaria, the Bavarian State Ministry of Food, Agriculture and Forestry, the Bavarian State Ministry of Health and Care (Health Initiative “Gesund.Leben.Bayern”), AOK Bayern – the largest statutory health insurance in Bavaria, the DEDIPAC consortium by the Joint Programming Initiative (JPI) “A Healthy Diet for a Healthy Life”, as well as the Technical University of Munich in the framework of the Open Access Publishing Program. Data collection, analysis, interpretation of data and manuscript preparation were independent from funders.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to data privacy/legal restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the Technical University of Munich (project number 5653/13). The study was conducted in accordance with the declaration of Helsinki. Participants provided written informed consent prior to study participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roxana Raab, Email: roxana.raab@tum.de.
Julia Hoffmann, Email: julia.hoffmann@tum.de.
Monika Spies, Email: monika.spies@tum.de.
Kristina Geyer, Email: k.geyer@tum.de.
Dorothy Meyer, Email: dora.meyer@tum.de.
Julia Günther, Email: julia.guenther@outlook.com.
Hans Hauner, Email: hans.hauner@tum.de.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO: Preterm birth. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed 09 August 2021.
- 4.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Berger R, Rath W, Abele H, Garnier Y, Kuon RJ, Maul H. Reducing the Risk of Preterm Birth by Ambulatory Risk Factor Management. Dtsch Arztebl Int. 2019;116(50):858–864. doi: 10.3238/arztebl.2019.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J. Born Too Soon Preterm Birth Action G: Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Bonney E, McElrath T, Lamont RF. Preterm Birth International c: Prevention of preterm birth: Proactive and reactive clinical practice-are we on the right track? Placenta. 2020;98:6–12. doi: 10.1016/j.placenta.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero DM, Larson J, Jacobsson B, Di Renzo GC, Norman JE, Martin JN, Jr, D'Alton M, Castelazo E, Howson CP, Sengpiel V, et al. Cross-Country Individual Participant Analysis of 4.1 Million Singleton Births in 5 Countries with Very High Human Development Index Confirms Known Associations but Provides No Biologic Explanation for 2/3 of All Preterm Births. PLoS One. 2016;11(9):e0162506. doi: 10.1371/journal.pone.0162506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO: WHO recommendations on antenatal care for a positive pregnancy experience. 2017. [PubMed]
- 11.EFCNI: https://www.efcni.org/activities/projects-2/escnh/. Accessed 09 August 2021.
- 12.Lindacher V, Altebaeumer P, Marlow N, Matthaeus V, Straszewski IN, Thiele N, Pfeil JM, Zimmermann LJI, Mader S. European Standards of Care for Newborn Health project m: European Standards of Care for Newborn Health-A project protocol. Acta Paediatr. 2021;110(5):1433–1438. doi: 10.1111/apa.15712. [DOI] [PubMed] [Google Scholar]
- 13.Chia AR, Tint MT, Han CY, Chen LW, Colega M, Aris IM, Chua MC, Tan KH, Yap F, Shek LP, et al. Adherence to a healthy eating index for pregnant women is associated with lower neonatal adiposity in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. Am J Clin Nutr. 2018;107(1):71–79. doi: 10.1093/ajcn/nqx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia AR, de Seymour JV, Colega M, Chen LW, Chan YH, Aris IM, Tint MT, Quah PL, Godfrey KM, Yap F, et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr. 2016;104(5):1416–1423. doi: 10.3945/ajcn.116.133892. [DOI] [PubMed] [Google Scholar]
- 15.Gete DG, Waller M, Mishra GD. Prepregnancy dietary patterns and risk of preterm birth and low birth weight: findings from the Australian Longitudinal Study on Women's Health. Am J Clin Nutr. 2020;111(5):1048–1058. doi: 10.1093/ajcn/nqaa057. [DOI] [PubMed] [Google Scholar]
- 16.Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal Dietary Patterns during the Second Trimester Are Associated with Preterm Birth. J Nutr. 2015;145(8):1857–1864. doi: 10.3945/jn.115.212019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haugen M, Meltzer HM, Brantsaeter AL, Mikkelsen T, Osterdal ML, Alexander J, Olsen SF, Bakketeig L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87(3):319–324. doi: 10.1080/00016340801899123. [DOI] [PubMed] [Google Scholar]
- 18.Hillesund ER, Overby NC, Engel SM, Klungsoyr K, Harmon QE, Haugen M, Bere E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa) Eur J Epidemiol. 2014;29(10):753–765. doi: 10.1007/s10654-014-9948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders L, Guldner L, Costet N, Kadhel P, Rouget F, Monfort C, Thome JP, Multigner L, Cordier S. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother-Child Cohort Study (TIMOUN) Paediatr Perinat Epidemiol. 2014;28(3):235–244. doi: 10.1111/ppe.12113. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen TB, Osterdal ML, Knudsen VK, Haugen M, Meltzer HM, Bakketeig L, Olsen SF. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87(3):325–330. doi: 10.1080/00016340801899347. [DOI] [PubMed] [Google Scholar]
- 21.Navarro P, Mehegan J, Murrin CM, Kelleher CC, Phillips CM. Adherence to the Healthy Eating Index-2015 across Generations Is Associated with Birth Outcomes and Weight Status at Age 5 in the Lifeways Cross-Generation Cohort Study. Nutrients. 2019;11(4):928. doi: 10.3390/nu11040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englund-Ogge L, Brantsaeter AL, Sengpiel V, Haugen M, Birgisdottir BE, Myhre R, Meltzer HM, Jacobsson B. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ. 2014;348:g1446. doi: 10.1136/bmj.g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai M, Zhang B, Yang R, Zheng T, Dong G, Lin H, Rigdon SE, Xian H, Hinyard L, Xaverius PK, et al. Association between maternal outdoor physical exercise and the risk of preterm birth: a case-control study in Wuhan, China. BMC Pregnancy Childbirth. 2021;21(1):206. doi: 10.1186/s12884-021-03678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domingues MR, Barros AJ, Matijasevich A. Leisure time physical activity during pregnancy and preterm birth in Brazil. Int J Gynaecol Obstet. 2008;103(1):9–15. doi: 10.1016/j.ijgo.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler S, Maxson P, Truong T, Swamy G. Psychosocial Stress and Preterm Birth: The Impact of Parity and Race. Matern Child Health J. 2018;22(10):1430–1435. doi: 10.1007/s10995-018-2523-0. [DOI] [PubMed] [Google Scholar]
- 26.Haviland MJ, Nillni YI, Cabral HJ, Fox MP, Wise LA, Burris HH, Hacker MR. Adverse psychosocial factors in pregnancy and preterm delivery. Paediatr Perinat Epidemiol. 2021;35(5):519–529. doi: 10.1111/ppe.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 28.Tanpradit K, Kaewkiattikun K. The Effect of Perceived Stress During Pregnancy on Preterm Birth. Int J Womens Health. 2020;12:287–293. doi: 10.2147/IJWH.S239138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Hedderson MM, Brown SD, Badon SE, Feng J, Quesenberry CP, Ferrara A. Healthy preconception and early-pregnancy lifestyle and risk of preterm birth: a prospective cohort study. Am J Clin Nutr. 2021;114(2):813–821. doi: 10.1093/ajcn/nqab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bindt C, Guo N, Bonle MT, Appiah-Poku J, Hinz R, Barthel D, Schoppen S, Feldt T, Barkmann C, Koffi M, et al. No association between antenatal common mental disorders in low-obstetric risk women and adverse birth outcomes in their offspring: results from the CDS study in Ghana and Cote D'Ivoire. PLoS One. 2013;8(11):e80711. doi: 10.1371/journal.pone.0080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghavan R, Dreibelbis C, Kingshipp BL, Wong YP, Abrams B, Gernand AD, Rasmussen KM, Siega-Riz AM, Stang J, Casavale KO, et al. Dietary patterns before and during pregnancy and birth outcomes: a systematic review. Am J Clin Nutr. 2019;109(Suppl_7):729S–756S. doi: 10.1093/ajcn/nqy353. [DOI] [PubMed] [Google Scholar]
- 32.Aune D, Schlesinger S, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of preterm birth: a systematic review and meta-analysis of epidemiological studies. BJOG. 2017;124(12):1816–1826. doi: 10.1111/1471-0528.14672. [DOI] [PubMed] [Google Scholar]
- 33.Rauh K, Kunath J, Rosenfeld E, Kick L, Ulm K, Hauner H. Healthy living in pregnancy: a cluster-randomized controlled trial to prevent excessive gestational weight gain - rationale and design of the GeliS study. BMC Pregnancy Childbirth. 2014;14:119. doi: 10.1186/1471-2393-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunath J, Gunther J, Rauh K, Hoffmann J, Stecher L, Rosenfeld E, Kick L, Ulm K, Hauner H. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care - the cluster-randomised GeliS trial. BMC Med. 2019;17(1):5. doi: 10.1186/s12916-018-1235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann J, Gunther J, Geyer K, Stecher L, Rauh K, Kunath J, Meyer D, Sitzberger C, Spies M, Rosenfeld E, et al. Effects of a lifestyle intervention in routine care on prenatal physical activity - findings from the cluster-randomised GeliS trial. BMC Pregnancy Childbirth. 2019;19(1):414. doi: 10.1186/s12884-019-2553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann J, Gunther J, Stecher L, Spies M, Geyer K, Raab R, Meyer D, Rauh K, Hauner H. Infant growth during the first year of life following a pregnancy lifestyle intervention in routine care-Findings from the cluster-randomised GeliS trial. Pediatr Obes. 2021;16(2):e12705. doi: 10.1111/ijpo.12705. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann J, Gunther J, Stecher L, Spies M, Meyer D, Kunath J, Raab R, Rauh K, Hauner H. Effects of a Lifestyle Intervention in Routine Care on Short- and Long-Term Maternal Weight Retention and Breastfeeding Behavior-12 Months Follow-up of the Cluster-Randomized GeliS Trial. J Clin Med. 2019;8(6):876. doi: 10.3390/jcm8060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunther J, Hoffmann J, Kunath J, Spies M, Meyer D, Stecher L, Rosenfeld E, Kick L, Rauh K, Hauner H. Effects of a Lifestyle Intervention in Routine Care on Prenatal Dietary Behavior-Findings from the Cluster-Randomized GeliS Trial. J Clin Med. 2019;8(7):960. doi: 10.3390/jcm8070960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johar H, Hoffmann J, Gunther J, Atasoy S, Stecher L, Spies M, Hauner H, Ladwig KH. Evaluation of antenatal risk factors for postpartum depression: a secondary cohort analysis of the cluster-randomised GeliS trial. BMC Med. 2020;18(1):227. doi: 10.1186/s12916-020-01679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunther J, Hoffmann J, Stecher L, Spies M, Geyer K, Raab R, Meyer D, Rauh K, Hauner H. How does antenatal lifestyle affect the risk for gestational diabetes mellitus? A secondary cohort analysis from the GeliS trial. Eur J Clin Nutr. 2021;76(1):150–158. doi: 10.1038/s41430-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21(6):521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Association of D. Pregnancy Study Groups Consensus P. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinwechter H, Schäfer-Graf U, Bührer C, Hoesli I, Kainer F, Kautzky-Willer A, Pawlowski B, Schunck K, Somville T, Sorger M. Gestationsdiabetes mellitus (GDM) –Diagnostik Therapie und Nachsorge. Diabetologie und Stoffwechsel. 2016;11:182–194. [Google Scholar]
- 44.Kuhn D-A. Entwicklung eines Index zur Bewertung der Ernährungsqualität in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1), German (“Development of a dietary quality index in the German Health Examination Survey for Adults”) Berlin: Robert Koch-Institut; 2017. [Google Scholar]
- 45.Koletzko B, Bauer CP, Bung P, Cremer M, Flothkotter M, Hellmers C, Kersting M, Krawinkel M, Przyrembel H, Rasenack R, et al. Nutrition in pregnancy - Practice recommendations of the Network "Healthy Start - Young Family Network". Dtsch Med Wochenschr. 2012;137(25–26):1366–1372. doi: 10.1055/s-0032-1305076. [DOI] [PubMed] [Google Scholar]
- 46.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 47.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 48.Rossi R, Murari A, Gaudio P, Gelfusa M. Upgrading Model Selection Criteria with Goodness of Fit Tests for Practical Applications. Entropy (Basel) 2020;22(4):447. doi: 10.3390/e22040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldenstrom U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017;124(8):1235–1244. doi: 10.1111/1471-0528.14368. [DOI] [PubMed] [Google Scholar]
- 50.Koullali B, van Zijl MD, Kazemier BM, Oudijk MA, Mol BWJ, Pajkrt E, Ravelli ACJ. The association between parity and spontaneous preterm birth: a population based study. BMC Pregnancy Childbirth. 2020;20(1):233. doi: 10.1186/s12884-020-02940-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph KS, Fahey J, Shankardass K, Allen VM, O'Campo P, Dodds L, Liston RM, Allen AC. Effects of socioeconomic position and clinical risk factors on spontaneous and iatrogenic preterm birth. BMC Pregnancy Childbirth. 2014;14:117. doi: 10.1186/1471-2393-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergstrom A, Charles MA, Chatzi L, Chevrier C, Chrousos GP, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European. North American and Australian cohorts BJOG. 2019;126(8):984–995. doi: 10.1111/1471-0528.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pigatti Silva F, Souza RT, Cecatti JG, Passini R, Jr, Tedesco RP, Lajos GJ, Nomura ML, Rehder PM, Dias TZ, Oliveira PF, et al. Role of Body Mass Index and gestational weight gain on preterm birth and adverse perinatal outcomes. Sci Rep. 2019;9(1):13093. doi: 10.1038/s41598-019-49704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avsar TS, McLeod H, Jackson L. Health outcomes of smoking during pregnancy and the postpartum period: an umbrella review. BMC Pregnancy Childbirth. 2021;21(1):254. doi: 10.1186/s12884-021-03729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geyer K, Spies M, Gunther J, Hoffmann J, Raab R, Meyer D, Rauh K, Hauner H. Effects of a Prenatal Lifestyle Intervention in Routine Care on Maternal Health Behaviour in the First Year Postpartum-Secondary Findings of the Cluster-Randomised GeliS Trial. Nutrients. 2021;13(4):1310. doi: 10.3390/nu13041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berghella V, Boelig R, Roman A, Burd J, Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2(4):100258. doi: 10.1016/j.ajogmf.2020.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harvey EM, McNeer E, McDonald MF, Shapiro-Mendoza CK, Dupont WD, Barfield W, Patrick SW. Association of Preterm Birth Rate With COVID-19 Statewide Stay-at-Home Orders in Tennessee. JAMA Pediatr. 2021;175(6):635–637. doi: 10.1001/jamapediatrics.2020.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedermann G, Hedley PL, Baekvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, Breindahl M, Melbye M, Hougaard DM, Christiansen M, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93–95. doi: 10.1136/archdischild-2020-319990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, O'Connell NH, Dunne CP. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a 'natural experiment' allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5(9):e003075. doi: 10.1136/bmjgh-2020-003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, van Dam RM, Chong MF. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv Nutr. 2019;10(4):685–695. doi: 10.1093/advances/nmy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gete DG, Waller M, Mishra GD. Effects of maternal diets on preterm birth and low birth weight: a systematic review. Br J Nutr. 2020;123(4):446–461. doi: 10.1017/S0007114519002897. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen MA, Maslova E, Halldorsson TI, Olsen SF. Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS One. 2014;9(4):e93644. doi: 10.1371/journal.pone.0093644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen J, Xun P, Chen C, Quan M, Wang R, Liu Y, He K. Non-occupational physical activity during pregnancy and the risk of preterm birth: a meta-analysis of observational and interventional studies. Sci Rep. 2017;7:44842. doi: 10.1038/srep44842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Currie LM, Woolcott CG, Fell DB, Armson BA, Dodds L. The association between physical activity and maternal and neonatal outcomes: a prospective cohort. Matern Child Health J. 2014;18(8):1823–1830. doi: 10.1007/s10995-013-1426-3. [DOI] [PubMed] [Google Scholar]
- 67.Leiferman JA, Evenson KR. The effect of regular leisure physical activity on birth outcomes. Matern Child Health J. 2003;7(1):59–64. doi: 10.1023/a:1022545718786. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann J, Gunther J, Geyer K, Stecher L, Kunath J, Meyer D, Spies M, Rosenfeld E, Kick L, Rauh K, et al. Associations between Prenatal Physical Activity and Neonatal and Obstetric Outcomes-A Secondary Analysis of the Cluster-Randomized GeliS Trial. J Clin Med. 2019;8(10):1735. doi: 10.3390/jcm8101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ACOG Committee Opinion No 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–e142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Preterm incidence in intervention and control group. Table S2 Characteristics of eligible and included participants. Table S3 Proportions of spontaneous and iatrogenic preterm births. Table S4 Associations between sociodemographic, health and lifestyle factors and the odds of preterm birth – models including linear covariates. Table S5 Associations between sociodemographic, health and lifestyle factors and the odds of iatrogenic preterm birth – models including categorical covariates. Table S6 Associations between sociodemographic, health and lifestyle factors and the odds of iatrogenic preterm birth – models including linear covariates. Table S7 Associations between sociodemographic, health and lifestyle factors and the odds of spontaneous preterm birth – models including categorical covariates. Table S8 Associations between sociodemographic, health and lifestyle factors and the odds of spontaneous preterm birth – models including linear covariates.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to data privacy/legal restrictions but are available from the corresponding author on reasonable request.