Figure 2.
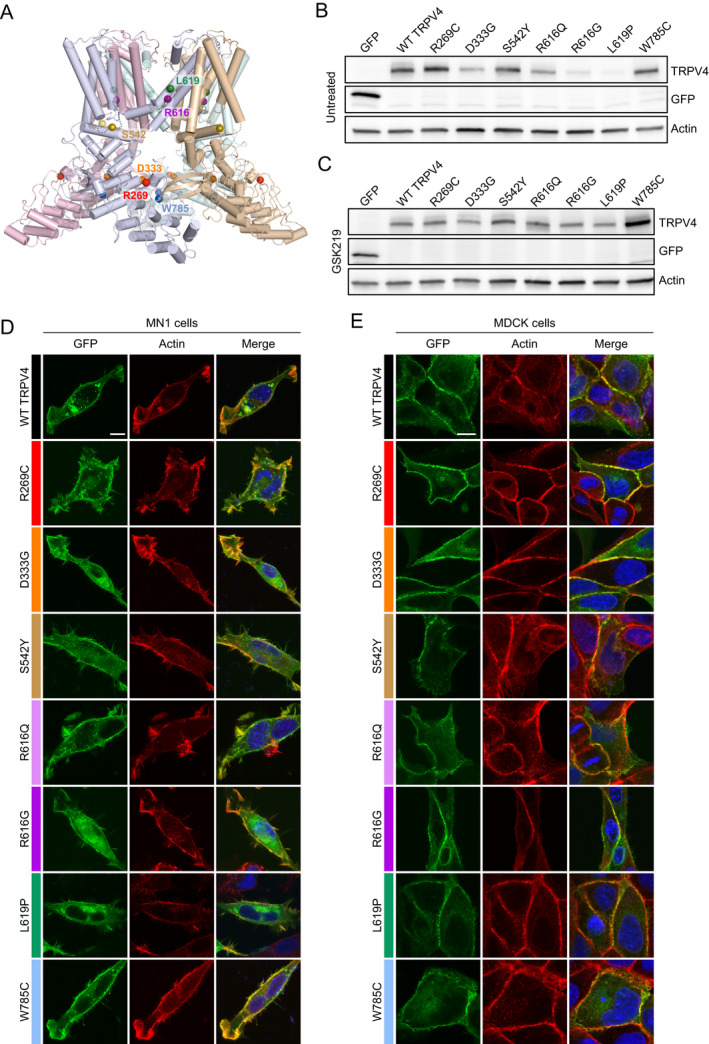
Expression and localization of selected TRPV4 mutants. (A) Cryo‐EM structure of the TRPV4 tetramer in an agonist‐bound state (PDB: 7AA5 47 ) depicting the location of mutated amino acid residues analyzed in vitro. Each monomer of the TRPV4 tetramer is displayed in a separate color. Colored spheres indicate the location of mutated amino acid residues within each monomer. (B) Immunoblot from HEK293T cells transfected with equal amounts of GFP‐tagged TRPV4 in the absence of TRPV4 antagonist. Expression of R616G and L619P appear reduced. (C) Immunoblot from HEK293T cells transfected with equal amounts of GFP‐tagged TRPV4 in the presence of 1 μM GSK219 TRPV4 antagonist. Expression of the individual mutants is normalized in the presence of antagonist. (D‐E) Immunohistochemistry of GFP‐tagged TRPV4 and actin (phalloidin) in MN‐1 cells (D) and MDCK cells (E) demonstrates normal trafficking to the cell membrane and co‐localization with cortical actin. [Colour figure can be viewed at wileyonlinelibrary.com]
