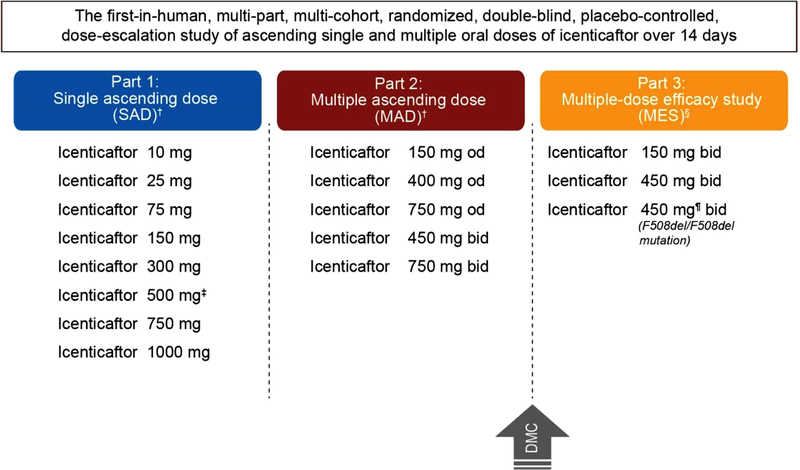
Fig. 1. Overall study overview and patient disposition.
† Healthy volunteers were randomized in to (3:1 ratio) for 14 days to icenticaftor (n = 6) or placebo (n = 2); ‡ Following completion of dosing, 5 out of 6 subjects received an additional dose for preliminary evaluation of effect of food on icenticaftor pharmacokinetics. Hence, the total number subjects remains 64; §icenticaftor was administered to patients with cystic fibrosis (Class III and IV or F508del/F508del mutation) for 14 days; icenticaftor 150 mg bid (n = 6), Placebo (n = 2); icenticaftor 450 mg bid(n = 12), Placebo (n = 4); icenticaftor 450 mg bid (F508del/F508del mutation, n = 19), Placebo (n = 6); ¶The planned enrolment in icenticaftor 450 mg bid was 32 patients who were homozygous for the F508del mutation; however, study enrolment was discontinued in this cohort based on results of interim analysis of 16 of 25 enrolled patients which suggested futility
bid, twice daily; DMC, data monitoring committee, MAD, multiple ascending dose; MES, multi-dose efficacy study; od, once daily; SAD, single ascending dose.