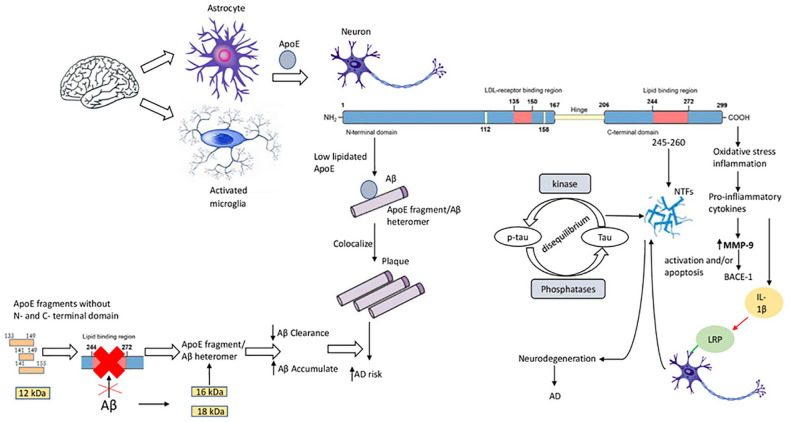
Figure 2.
The role of apolipoprotein E (ApoE) peptides in neurodegeneration and Alzheimer’s disease (AD). In the brain, astrocytes and activated microglia synthesize ApoE. In neurons, ApoE is cleaved in the C-terminal domain that binds to amyloid β (Aβ) and localizes to plaques; and in the N-terminal domain that localizes with NFTs. Low ApoE lipidation influences the binding of ApoE4 to Aβ, promoting ApoE-fragment/Aβ heteromer generation. ApoE fragments without N- and C-terminal domains (ApoE 133-149, ApoE 141-149, ApoE 141-155, 12 kDa fragment) do not have the Aβ transporter-binding domain – these fragments (together with Aβ accumulation) favor the formation of ApoE-fragment/Aβ heteromers, decelerating Aβ clearance and favoring Aβ accumulation in AD. The lipidated form of ApoE interacts with NFTs through its amino acid residues 245-260. Imbalance between the activities of tau protein kinases and phosphatases promotes accumulation of tau protein in neurons. C-terminal truncated ApoE fragments induce oxidative stress and inflammation, releasing pro-inflammatory cytokines. Increased MMP-9 leads to β-site amyloid precursor protein–cleaving enzyme-1 (BACE-1) activation and/or apoptosis, and interleukin (IL)-1β interacting with neurons by LDL-related protein receptor induces NFT formation.