Figure 6. Chemical induction of R15 expression inhibits signaling and growth of human tumor lines driven by select RAS mutations.
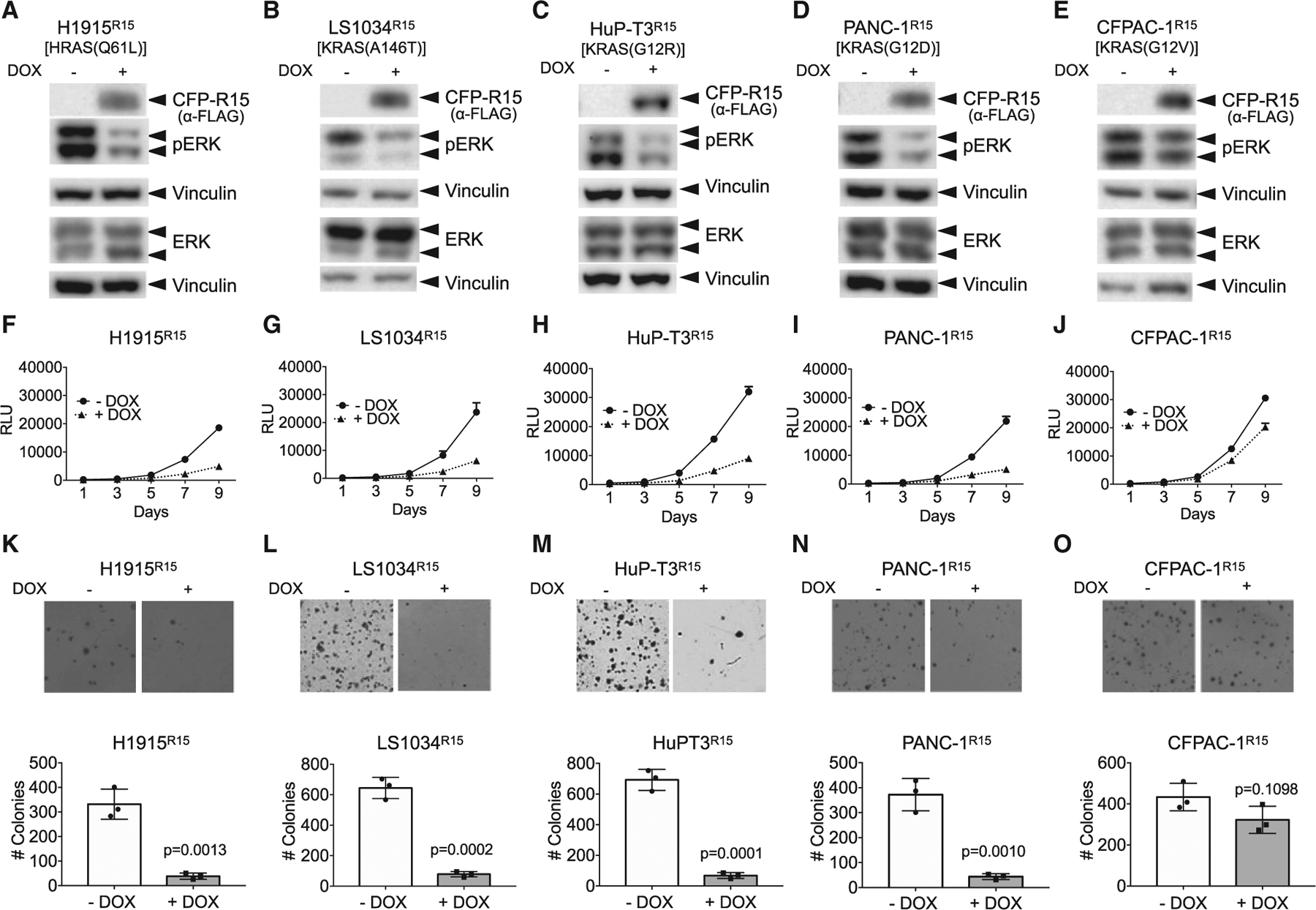
(A–E) Doxycycline (DOX)-inducible, R15-expressing stable lines were generated from RAS mutant tumor cells. ERK-MAPK activation was then measured ± DOX treatment by western blot analysis for pERK levels. The mutant RAS protein expressed in each tumor line is indicated above the panels. Vinculin expression was used as a control for loading.
(A, F, and K) H1915R15; (B, G, and L) LS1034R15; (C, H, and M) HuPT3R15; (D, I, and N) PANC-1R15; and (E, J, and O) CFPAC-1R15. Data from additional tumor lines are shown in Figure S4. The experiments were repeated three times for each cell line except H1944R15 and A375R15, which were repeated two times.
(F–J) R15 expression reduced the proliferation of a subset of RAS mutant human tumor cells. Results are the average of triplicate wells ± SEM shown by bars.
(K–O) R15 expression reduced anchorage-independent growth of a subset of RAS mutant human tumor cells. Engineered R15 cells were plated on soft agar in the absence (−) or presence (+) of DOX and allowed to grow for 3 to 4 weeks. Graphs represent the average colony number from three wells ± SD. Colonies were counted using NIH ImageJ software. Images are representative wells from each assay. The experiments were done in three technical replicates for each cell line. p values were determined by comparison of colony numbers between −DOX and +DOX conditions using a Student’s t test. Data on additional lines are shown in Figure S4. See Figure S5 for data on the effects of R15 on AKT activation.
