FIGURE 5.
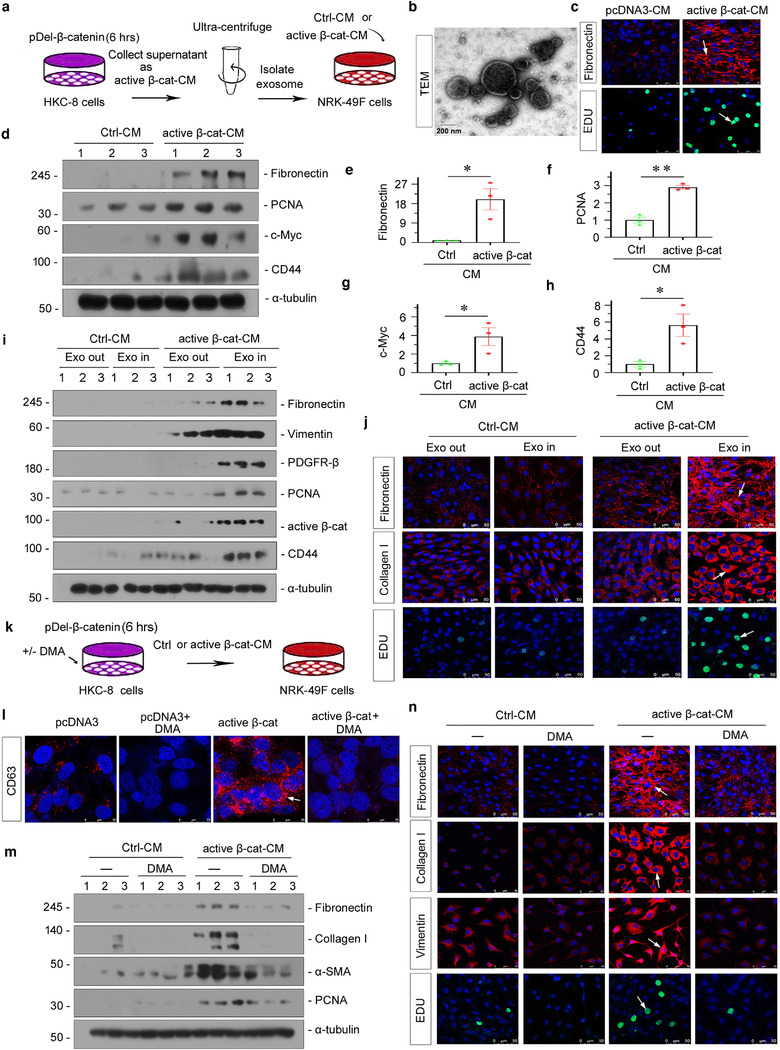
Tubular cell‐derived exosomes controlled by β‐catenin promote fibroblast activation in vitro. (a) Experimental design. Human kidney proximal tubular epithelial cells (HKC‐8) were transfected with active β‐catenin expression plasmid (pDel‐β‐catenin) for 6 h and then washed and continued to be incubated for an additional 24 h in serum‐free medium (active β‐cat conditioned medium). Exosomes were isolated from conditioned media by ultracentrifugation. Conditioned media from HKC‐8 cells were collected and used to stimulate normal rat kidney interstitial fibroblasts (NRK‐49F). (b) TEM showing the exosomes isolated from the conditioned media of HKC‐8 cells; scale bar: 200 nm. (c–j) Conditioned media from HKC‐8 cells that were transfected with active β‐catenin plasmid could stimulate the activation in NRK‐49F cells in vitro. NRK‐49F cells were treated with 40% of active β‐cat conditioned medium for 24 h. (c) Representative micrographs showing immunofluorescence staining of fibronectin and EDU staining in NRK‐49F cells after incubation with conditioned media from HKC‐8 cells. Arrow indicates positive staining; scale bar: 50 μm. (d–h) Representative western blot (d) and quantitative data (e–h) show that the expression of fibronectin (e), PCNA (f), c‐Myc (g) and CD44 (h) was induced in NRK‐49F cells by conditioned media from HKC‐8 cells. *p < 0.05, **p < 0.01 versus pcDNA3‐CM; numbers (1 to 3) indicate individual treatment in a given group. (i, j) Exosomes are necessary to mediate the activation in fibroblasts induced by conditioned media from HKC‐8 cell. (i) Western blot analyses show that the conditioned media lacking exosomes from HKC‐8 cells failed to induce fibronectin, vimentin, PDGFR‐β, PCNA, active β‐catenin and CD44 expression in NRK‐49F cells. (j) Representative micrographs showing immunofluorescence staining of fibronectin (upper), collagen I (middle), and EDU staining (bottom) in different groups, as indicated. Arrow indicates positive staining; scale bar: 50 μm. (k) HKC‐8 cells were transfected with pDel‐β‐catenin for 6 h in the absence or presence of dimethyl amiloride (DMA),and continued to be incubated for additional 24 h in serum‐free medium. Conditioned media were collected and used to stimulate NRK‐49F cells. HKC‐8 cells were pretreated with 100 μmol/L of DMA for 1 h, and transfected with pDel‐β‐catenin plasmid for 6 h, and then incubated with serum‐free medium for 24 h (active β‐cat conditioned medium). NRK‐49F cells were treated with 40% of active β‐cat conditioned medium for 24 h. (l) Immunofluorescence staining of CD63 shows that DMA, an exosome inhibitor, inhibited exosome secretion. Representative immunofluorescence staining micrographs of CD63 are shown, as indicated. Arrow indicates positive staining; scale bar: 50 μm. (m) Western blot analyses show that the blockade of exosome generation by DMA inhibited the expression of fibronectin, collagen I, α‐SMA and PCNA in NRK‐49F cells induced by conditioned media from HKC‐8. (n) Representative micrographs of immunofluorescence staining of fibronectin, collagen I, vimentin and EDU in NRK‐49F cells after incubation with conditioned media, as indicated. Arrow indicates positive staining; scale bar: 50 μm
