FIGURE 6.
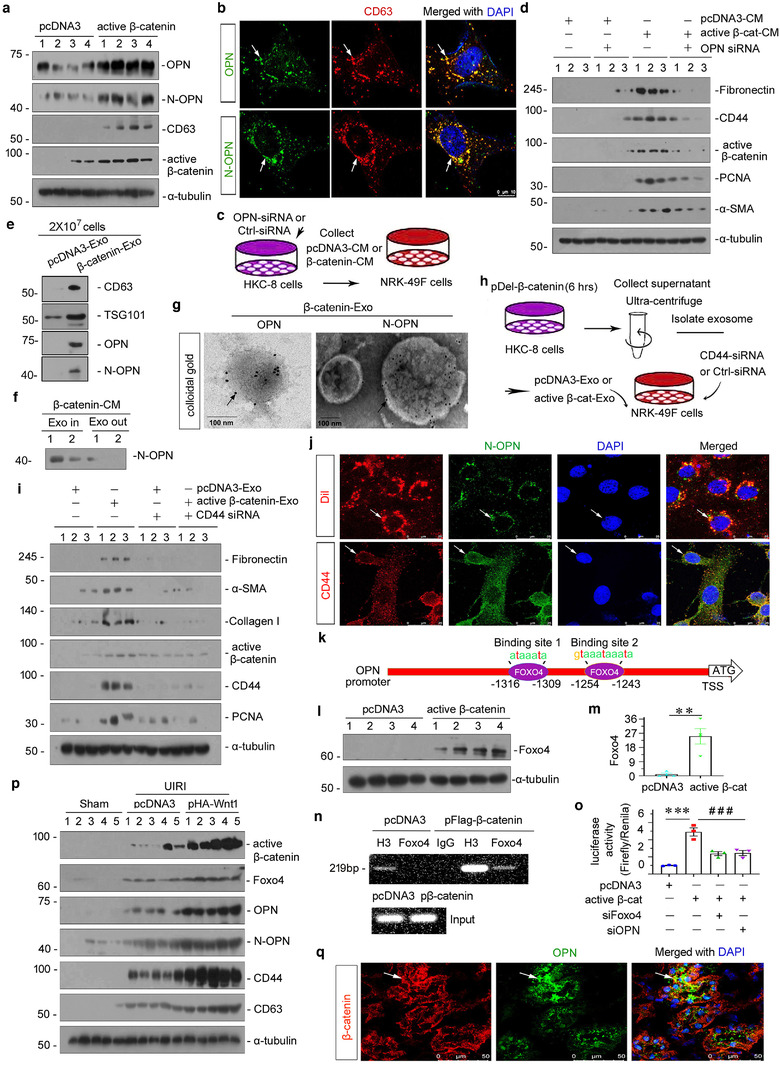
Exosome‐mediated activation of the OPN/CD44 axis in tubule‐fibroblast communication is controlled by β‐catenin. (a) Western blot analyses showing protein expression of OPN, N‐OPN, CD63 and active β‐catenin in Human kidney proximal tubular (HKC)‐8 cells transfected with pDel‐β‐catenin for 24 h. Numbers (1 to 4) indicate individual treatment in a given group. (b) Colocalization of CD63 and OPN (upper), and CD63 and N‐OPN (bottom) is shown by double immunofluorescence staining in HKC‐8 cells transfected with pDel‐β‐catenin for 24 h. Arrow indicates positive staining; scale bar: 10 μm. (c) Experimental design shows OPN was knocked down in HKC‐8 cells prior to the collection of conditioned media. HKC‐8 cells were cotransfected with siRNA to OPN and pDel‐β‐catenin plasmid for 6 h, and then incubated with serum‐free medium for 24 h (active β‐cat conditioned medium). NRK‐49F cells were treated with 40% of conditioned medium for 24 h. (d) Western blot shows that knockdown of OPN in HKC‐8 cells abolished the expression of fibronectin, CD44, active‐β‐catenin, PCNA and α‐SMA in NRK‐49F cells after incubation with conditioned media. Numbers (1 to 3) indicate individual treatment in a given group. (e) Western blot analyses demonstrate the presence of CD63, TSG101, OPN and N‐OPN proteins in the exosomes isolated from HKC‐8 cells transfected with pDel‐β‐catenin. Exosomes prepared from the same amounts of HKC‐8 cells transfected with pDel‐β‐catenin were lysed and immunoblotted with antibodies against CD63 and TSG101, OPN and N‐OPN. HKC‐8 cells were transfected with pDel‐β‐catenin plasmid for 6 h, and then incubated with serum‐free medium for 24 h. Supernatant was collected and used to isolate exosomes. (f) Western blot analyses show N‐OPN protein expression in supernatant and exosome‐removed supernatant in HKC‐8 cells transfected with pDel‐β‐catenin. HKC‐8 cells were transfected with pDel‐β‐catenin plasmid for 6 h, and then incubated with serum‐free medium for 24 h (active β‐cat conditioned medium). Exosomes were removed or not in active β‐cat conditioned medium. (g) Colloidal gold staining demonstrates that both OPN and N‐OPN were encapsulated by exosomes isolated from HKC‐8 cells transfected with pDel‐β‐catenin. Both OPN and N‐OPN were labelled with 10 nm colloidal gold. Arrows indicate OPN and N‐OPN, respectively; scale bar: 100 nm. (h) Experimental design show CD44 was knocked down in NRK‐49F fibroblasts prior to stimulation with tubule‐derived exosomes. HKC‐8 cells were transfected with pDel‐β‐catenin plasmid or pcDNA3 for 6 h, and then incubated with serum‐free medium for 24 h. Supernatant was collected and used to isolate exosomes (active β‐catenin‐Exo or pcDNA3‐Exo). NRK‐49F cells were transfected with siRNA to CD44 for 6 h, and then treated with active β‐catenin‐Exo or pcDNA3‐Exo (30 μg/ml) for 24 h. (i) Western blot analyses show that knockdown of CD44 in NRK‐49F fibroblast cells abolished the expression of fibronectin, α‐SMA, collagen I, active β‐catenin, CD44 and PCNA in NRK‐49F cells after incubation with tubular cell‐derived exosomes (active β‐catenin‐Exo). (j) Double fluorescent staining confirms the intracellular transfer of tubule‐derived exosomal N‐OPN and its colocalization with the receptor of CD44 in NRK‐49F cells. HKC‐8 cells were transfected with pDel‐β‐catenin plasmid for 6 h, and then incubated with serum‐free medium for 24 h. Supernatants were used to isolate exosomes, and incubated with or without Dil (red). HKC‐8 cell‐derived exosomes (30 μg/ml) were incubated into NRK‐49F cells for 12 h, followed by immunofluorescence staining for N‐OPN (green) or CD44 (red) in NRK‐49F cells. Arrows indicate HKC‐8 cell‐derived exosomes and positive staining. Scale bar, 25 μm. (k) Schematic diagram showing the binding site of Foxo4 with OPN. (l, m) Western blot analyses showing the upregulation of Foxo4 protein in HKC‐8 cells transfected with pDel‐β‐catenin. Representative western blot (l) and quantitative data (m) are shown. Numbers (1 to 4) indicate individual treatment in a given group. HCK‐8 cells were transfected with pDel‐β‐catenin plasmid or pcDNA3 for 24 h. **p < 0.01 versus pcDNA3 (n = 4). (n) Representative chromatin immunoprecipitation (chip) assay results showing the binding of Foxo4 to the OPN gene promoter region. HKC‐8 cells were transfected with pDel‐β‐catenin or pcDNA3 for 24 h. Cell lysates were precipitated with an antibody against Foxo4, histone H3, or nonimmune IgG, and the ChIP assay was performed for OPN gene promoters. Total diluted lysate was used as the total genomic input DNA. (O) Representative luciferase assay results showing that β‐catenin augmented OPN targeted gene transcription in a Foxo4‐dependent manner. HKC‐8 cells were cotransfected with renilla, pGL3‐OPN, pDel‐β‐catenin, Foxo4 siRNA or OPN siRNA, as indicated. ***p < 0.001 versus pcDNA3 controls, ### p < 0.001 versus pDel‐β‐catenin plus Foxo4 siRNA or pDel‐β‐catenin plus OPN siRNA (n = 3). (p) Representative western blot showing the expression of active β‐catenin, Foxo4, OPN, N‐OPN and CD44. At 4 days after surgery, UIRI mice were injected with empty vector (pcDNA3) or Wnt1 expression vector (pHA‐Wnt1) through hydrodynamic‐based gene delivery. (q) Colocalization of β‐catenin and OPN are shown using double immunofluorescence staining in Wnt1 overexpressed mice after UIRI. Arrow indicates positive staining; scale bar: 50 μm
