Abstract
Background
Racial and ethnic minorities experience well-documented disparities across the cancer trajectory. However, factors underlying these disparities may vary regionally. The Health Belief Model (HBM) was developed to explain and predict health-related prevention and early detection behaviors, particularly uptake of health services. Our goal was to use the HBM to guide an exploration of factors that contribute to racial/ethnic health disparities in the catchment area of a large National Cancer Institute-designated Comprehensive Cancer Center in the Southeastern United States.
Methods
We conducted a secondary analysis of data collected by the cancer center for its triennial Community Health Needs Assessment, which sampled adults from the center’s 15-county catchment area. White non-Hispanics (WNHs; n = 887), Black non-Hispanics (BNHs; n = 78), Hispanics/Latinxs (H/Ls; n = 185), and those identifying as another race/ethnicity (“Others”; n = 39) were compared across key HBM variables, including demographic/psychosocial information, perceived benefits and barriers to preventive health behaviors, risk perception, and health behavior outcomes.
Results
Controlling for annual household income, relationship status, and age (for certain screening behaviors), significant differences were seen in information-seeking behaviors, risk perception, community attributes, discrimination, and distress. Non-WNH groups reported worse community attributes, higher everyday discrimination, lower health literacy, less confidence in their ability to get health information, and lower perceived risk of cancer.
Conclusion
This analysis presents a better understanding of how HBM factors may influence health disparities in the cancer center’s catchment area. Results describe the needs of community members from racial and ethnic minority groups, which will inform future research, education, outreach, and service activities.
Keywords: Cancer, Catchment area, Minority health, Health behavior, Health disparities
Introduction
Cancer health disparities are defined by the National Cancer Institute (NCI) as differences in the incidence, prevalence, mortality, and burden of cancer and related adverse health conditions that exist among specific population groups in the United States [1]. Cancer health disparities are well-documented for racial and ethnic minority groups across the cancer continuum [2] and often reflect broader health inequalities [3]. For example, racial and ethnic minorities experience barriers in access to cancer screening and prevention services [4, 5] and treatment options, [6] including enrollment in clinical trials [7]. Given these barriers, it is unsurprising that patients belonging to racial and ethnic minority groups also have worse survival [8, 9] and report lower quality of life [10, 11].
Addressing these disparities is a research priority in the United States and globally [2, 12]. However, the factors underlying these disparities can vary geographically; for example, there is geographical variation in cancer risk-reducing health behaviors, such as HPV vaccination and colorectal cancer screening [13, 14]. Past research suggests that patient-level health beliefs, practices, and preferences are non-negligible factors determining geographic variation in health care [15]. As such, a one-size-fits-all approach likely will fail to fully address cancer health disparities at the national or state level, and a more nuanced assessment of local individual-level beliefs and behaviors is needed. One model that can guide the exploration of patient-level beliefs and behaviors that may impact health disparities is the Health Belief Model (HBM; Fig. 1). This model aims to explain and predict health-related action and uptake of health services depending on a variety of constructs, including perceived susceptibility to and severity of illness/disease, benefits of and barriers to engaging in health-related actions, and self-efficacy to perform those actions [16]. The HBM has been used extensively to identify key barriers and develop interventions for health behavior change or service use in general and more specifically in addressing and understanding health disparities. Among racial and ethnic minority populations, the HBM has been used to understand beliefs about cancer prevention to help guide design and implementation of interventions [17], understand beliefs about cervical cancer screening [18], increase clinical trial participation [19], and understand health-seeking behaviors for mental health services [20]. The HBM is a useful theoretical framework for its potential to identify sociocultural attitudes and beliefs, specific to racial/ethnic subgroups, which may influence various behaviors related to cancer screening and prevention strategies.
Fig. 1.
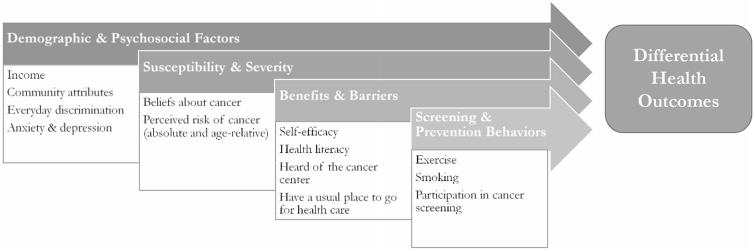
Adapted Health Belief Model with variables used in the current study
The NCI Cancer Centers program sets the expectation that cancer centers engage in both research and outreach to reduce both the overall cancer burden and disparities in the community they serve (i.e., the catchment area) [21]. As such, our goal was to use the HBM to guide an exploration of factors that may contribute to disparities in care for racial and ethnic minority groups in one NCI-designated Comprehensive Cancer Center’s catchment area, including demographic/psychosocial factors, perceived benefits of and barriers to health behaviors, risk perception, and health behavior outcomes.
Methods
This was a secondary analysis of cross-sectional self-report data collected by a large NCI-designated Comprehensive Cancer Center in the Southeastern United States in February 2019 for its triennial Community Health Needs Assessment. The cancer center utilizes this assessment to address health disparities for both the institution as a whole [22] and, more recently, as a tool to guide research and outreach efforts. This study was determined by the Institutional Review Board not to be human subjects research and, was therefore, exempt from review.
Participants and procedure
The cancer center’s catchment area (Fig. 2) includes approximately 6.1 million people from 15 of Florida’s 67 counties in West Central Florida, accounting for 29% of all state residents and 34% of all cancer cases. Blacks and Hispanics comprise the largest minority groups in our area, accounting for 11% and 17% of the population, respectively.
Fig. 2.
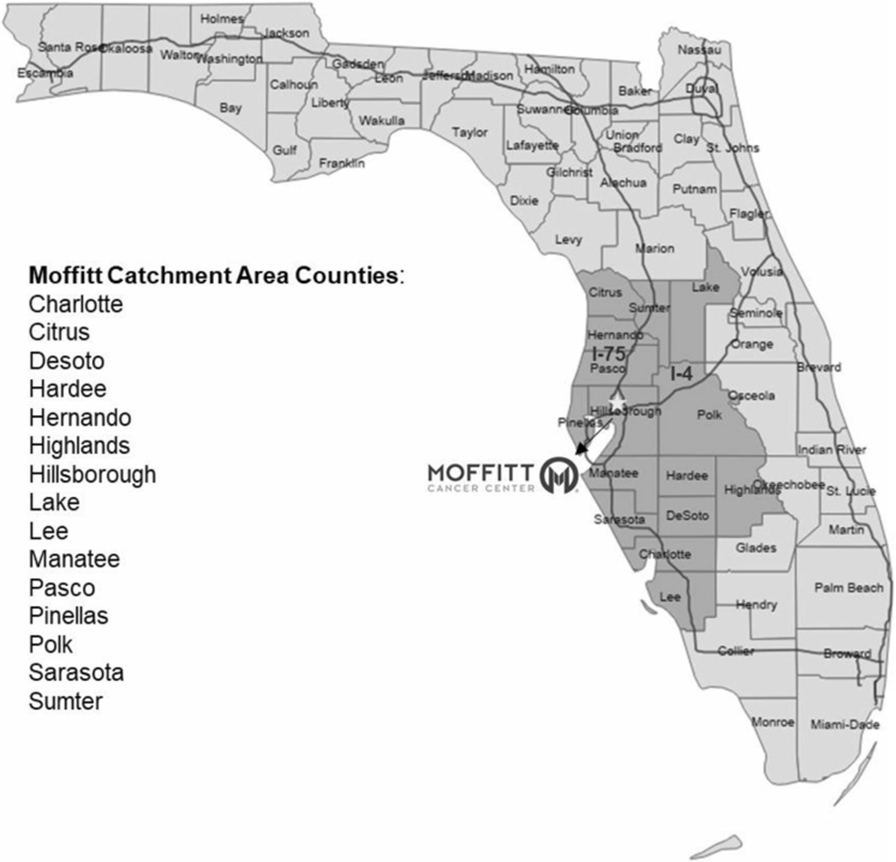
Moffitt Cancer Center’s catchment area
In February 2019, we conducted an online survey with a sample of adults from the cancer center’s catchment area using the YouGov opt-in panel (https://today.yougov.com/opi/). YouGov, a strategic marketing consulting and research services firm (Palo Alto, California, USA), maintains a consumer panel of 1.9 million adults in the United States recruited through web-based advertising campaigns, online advertisements, and mail, telephone, and web recruitment campaigns. The YouGov panel is large and demographically diverse, which allows for sample matching, a model-based approach to non-probability-based sampling.
YouGov constructs representative samples with a two-stage sampling design. First, a sample frame is constructed by combining individual-level data from the United States Census American Community Survey and the Bureau of Labor Statistics. YouGov then draws a stratified random sample of people that is similar in size to the desired study sample, and the sampling algorithm behind the proprietary sampling system searches the opt-in panel for participants who most closely match the individuals in the randomly drawn target sample. The sampling algorithm for the Community Health Needs Assessment was built upon matches by age, race/ethnicity, income, and education for every respondent in the target frame. YouGov oversampled racial/ethnic minorities in the bigger counties in the catchment area with larger minority populations (i.e., Hillsborough, Pinellas, Pasco, and Polk). Additionally, the survey link was shared with leaders of three catchment area community groups to distribute.
Measures
The online self-report questionnaire was modeled in part after NCI’s Population Health in Cancer Center Catchment Area survey [23] and adapted in collaboration with an expert group of population science researchers, reviewed, and conducted a soft launch to ensure data was collected appropriately. The questionnaire captured demographics, personal health behaviors and beliefs, access to healthcare, and community perceptions and attributes. As outlined below, existing measures from this study were used as proxies for domains of the HBM: perceived susceptibility and severity; perceived benefits, barriers, and self-efficacy; and cues to action (screening and prevention behaviors).
Race and ethnicity
The questionnaire included one item asking participants to self-identify their race; respondents were permitted to select multiple racial categories. The questionnaire also included one item asking participants to self-identify their ethnicity—either Hispanic/Latinx or non-Hispanic/Latinx. Participants were coded as one of four race/ethnicity categories based on their responses to these two items: WNH (White non-Hispanic/Latinx), BNH (Black non-Hispanic/Latinx), H/L (Hispanic/Latinx of any race), or ONH (non-Hispanic/Latinx and any other race, including American Indian, Asian, Native Hawaiian/Pacific Islander, and “other”).
Other demographic and psychosocial factors
Participants self-reported their age in years, annual household income using categories ranging from $0 to $9,999 to $100,000 or more, and relationship status using the categories married/domestic partner, living as married, divorced, widowed, separated, and single/never married. Relationship status was re-coded into a dichotomous variable for analyses: married/domestic partner and living as married were combined into a single partnered category, and the remaining relationship status categories were combined into a single unpartnered category.
Community attributes
Community attributes were assessed using a composite of 11 items regarding participants’ perceptions of their neighborhood or community. Items consisted of statements pertained to drug/alcohol abuse, parks and facilities, availability of jobs, seriousness of crime, air pollution, safety, housing affordability, quality of health care, presence of safe sidewalks, and availability of healthy foods. Each item solicited level of agreement on a Likert-type scale ranging from 1 (strongly agree) to 5 (strongly disagree). Items were reverse-scored as necessary and then averaged, with a higher overall score indicating worse perception of community attributes.
Everyday discrimination
Everyday discrimination was based on five Likert-type items regarding everyday treatment by others (e.g., “People act as if they think you are not smart,” “You are threatened or harassed”). Participants rated the frequency of these items from 1 (never) to 5 (at least once a week). The composite score was an average of the responses for each item, with higher scores indicating a greater frequency of discrimination in daily life.
Six items were used to assess symptoms of anxiety and depression over the past month. Responses were given on a Likert-type scale ranging from 1 (none of the time) to 5 (all of the time), with higher scores indicative of worse symptoms. Responses to the three anxiety items were averaged to create a composite anxiety symptoms variable, and responses to the three depression items were averaged to create a composite depression symptoms variable.
Susceptibility and severity
Perceived susceptibility, or perceived likelihood of developing or contracting a disease, and perceived severity, or feelings regarding medical and social consequences of disease, are collectively labeled as a threat in the HBM [24]. Perceived control over cancer risk was assessed with five items (e.g., “It seems like everything causes cancer,” “Cancer is most often caused by a person’s behavior or lifestyle”). Responses were given on a Likert-type scale ranging from 1 (strongly agree) to 4 (strongly disagree) and then averaged to create a composite variable called beliefs about cancer, with higher scores representing greater perceived control over cancer risk. Perceived personal risk of cancer was assessed using two items. One item assessed perceived absolute risk of cancer on a scale of 0 (no chance of getting cancer) to 100 (will definitely get cancer). The second item asked, “Compared to other people your age, how likely are you to get cancer in your lifetime?” (perceived age-relative risk of cancer); responses were given on a Likert-type scale ranging from 1 (much less likely) to 5 (much more likely).
Benefits and barriers to preventive health behaviors
A single item assessed self-efficacy, or how confident participants were that they could get advice or information about health and medical topics if needed; responses were rated on a Likert-type scale ranging from 1 (completely confident) to 5 (not confident at all), with higher scores indicating worse self-efficacy. A single item assessed health literacy, operationalized as frequency of needing help in understanding written material from providers, clinics, or pharmacies, with responses on a Likert-type scale ranging from 1 (always) to 5 (never), with higher scores indicating better health literacy. Participants were also asked if they had ever heard of the cancer center (yes/no), and if they had a place they usually go (place for care) when they are sick or need advice about their health (yes/no).
Screening and prevention behaviors
Exercise frequency was assessed with a single item asking participants to estimate how many days they participate in moderate-intensity physical activity/exercise during a typical week (days of exercise per week). Smoking history was captured with a single yes/no item: “Have you smoked at least 100 cigarettes in your entire life?”. Engagement in cancer screening/prevention services (yes/no) was assessed with three items which asked whether the participant had ever had a blood test for hepatitis C (a known risk factor for liver and other cancers), whether a provider had ever recommended an HPV vaccine for the participant or a family member, and whether a provider had ever recommended colorectal cancer screening. Female-identifying participants were also asked whether they had ever (yes/no) had a mammogram, Pap test, and/or HPV test.
Data analysis
Descriptive statistics were calculated for demographic variables and key HBM variables. Initial comparisons on demographic variables showed significant differences between racial/ethnic groups in annual household income (F(3, 1083) = 5.41, p = 0.001) and relationship status (i.e., partnered vs. unpartnered; χ2(3) = 13.68, p = 0.003). Therefore, analysis of covariance/multivariate analysis of covariance (ANCOVA/MANCOVA) and logistic regression (for dichotomous outcomes) were conducted to compare racial/ethnic groups on key HBM variables, controlling for annual household income and relationship status. Additionally, age was included as a covariate for certain prevention and screening-related health behaviors (i.e., recommended HPV vaccine, recommended colorectal cancer screening, mammogram).
Results
Demographics and descriptive statistics
Responses were recorded from a total of 1196 individuals (887 WNHs, 78 BNHs, 185 H/Ls, 39 ONHs, and seven individuals who did not report race/ethnicity information; national survey n = 1000, community response n = 196). See Table 1 for demographic characteristics by racial/ethnic group.
Table 1.
Sample demogr aphics
| Variable | All (N = 1196) |
WNH (n = 887) |
BNH (n = 78) | H/L (n = 185) | ONH (n = 39) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| n | % | n | % | n | % | n | % | n | % | |
| Age | 53.19 | 53.19 | 56.07 | 15.98 | 47.10 | 16.13 | 43.27 | 15.67 | 15.67 | 14.44 |
| Gender | ||||||||||
| Male | 464 | 38.8 | 333 | 37.5 | 37 | 47.4 | 70 | 37.8 | 22 | 56.4 |
| Female | 731 | 61.1 | 554 | 62.5 | 41 | 52.6 | 115 | 62.2 | 17 | 43.6 |
| Prefer not to answer | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | ||||
| Sexual orientation | ||||||||||
| Straight/heterosexual | 1093 | 91.4 | 821 | 92.6 | 74 | 94.9 | 159 | 85.9 | 33 | 84.6 |
| Gay or lesbian | 44 | 3.7 | 31 | 3.5 | 2 | 2.6 | 7 | 3.8 | 4 | 10.3 |
| Bisexual | 36 | 3.0 | 26 | 2.9 | 1 | 1.3 | 8 | 4.3 | 1 | 2.6 |
| Prefer to self-describe | 2 | 0.2 | 1 | 0.1 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| Prefer not to answer | 21 | 1.8 | 8 | 0.9 | 1 | 1.3 | 10 | 5.4 | 1 | 2.6 |
| Household income | ||||||||||
| $0–$9,999 | 39 | 3.3 | 16 | 1.8 | 7 | 9.0 | 15 | 8.1 | 1 | 2.6 |
| $10,000–$19,999 | 83 | 6.9 | 64 | 7.2 | 5 | 6.4 | 7 | 3.8 | 7 | 17.9 |
| $20,000–$34,999 | 177 | 14.8 | 117 | 13.2 | 16 | 20.5 | 40 | 21.6 | 4 | 10.3 |
| $35,000–$49,999 | 170 | 14.2 | 127 | 14.3 | 11 | 14.1 | 26 | 14.1 | 5 | 12.8 |
| $50,000–$74,999 | 261 | 21.8 | 209 | 23.6 | 11 | 14.1 | 34 | 18.4 | 5 | 12.8 |
| $75,000–$99,999 | 144 | 12.0 | 115 | 13.0 | 5 | 6.4 | 19 | 10.3 | 5 | 12.8 |
| $10,0000+ | 217 | 18.1 | 164 | 18.5 | 14 | 17.9 | 26 | 14.1 | 11 | 28.2 |
| Don’t know | 14 | 1.2 | 4 | 0.5 | 3 | 3.8 | 7 | 3.8 | 0 | 0.0 |
| Prefer not to answer | 91 | 7.6 | 71 | 8.0 | 6 | 7.7 | 11 | 5.9 | 1 | 2.6 |
| Marital status | ||||||||||
| Married/domestic partner | 647 | 54.1 | 510 | 57.5 | 29 | 37.2 | 88 | 47.6 | 18 | 46.2 |
| Living as married | 68 | 5.7 | 45 | 5.1 | 4 | 5.1 | 18 | 9.7 | 1 | 2.6 |
| Divorced | 143 | 12.0 | 114 | 12.9 | 7 | 9.0 | 17 | 9.2 | 4 | 10.3 |
| Widowed | 74 | 6.2 | 65 | 7.3 | 3 | 3.8 | 4 | 2.2 | 2 | 5.1 |
| Separated | 19 | 1.6 | 11 | 1.2 | 4 | 5.1 | 3 | 1.6 | 1 | 2.6 |
| Single, never married | 237 | 19.8 | 141 | 15.9 | 29 | 37.2 | 53 | 28.6 | 13 | 33.3 |
| Prefer not to answer | 8 | 0.7 | 1 | 0.1 | 2 | 2.6 | 2 | 1.1 | 0 | 0.0 |
WNH White non-Hispanic/Latinx, BNH Black non-Hispanic/Latinx, H/L Hispanic/Latinx of any race, ONH other non-Hispanic/Latinx and belonging to a race other than White or Black (e.g., American Indian, Asian)
Health belief model analyses
Table 2 shows descriptive statistics for key HBM variables, as well as results of (M)ANCOVA and logistic regression analyses. Significant differences between racial/ethnic groups were seen across several key HBM domains.
Table 2.
Descriptive statistics for community health needs assessment items organized by HBM construct, and results of (M)ANCOVA and logistic regression analyses
| HBM construct | Community health needs assessment variable | All (N = 1196) |
WNH (n = 887) |
BNH (n = 78) |
H/L (n = 185) |
ONH (n = 39) |
Group differences |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | F | p | ||
| Demographic/Psycho-social | Community attributes | 2.76 | 0.76 | 2.67 | 0.65 | 3.06 | 1.09 | 3.03 | 0.99 | 2.94 | 0.67 | 9.36 | < .001 |
| Demographic/Psycho-social | Everyday discrimination | 1.72 | 0.77 | 1.66 | 0.70 | 2.06 | 1.05 | 1.82 | 0.85 | 2.04 | 1.01 | 9.23 | < .001 |
| Demographic/Psycho-social | Distress – Anxiety | 4.08 | 0.97 | 4.14 | 0.92 | 3.85 | 1.17 | 3.88 | 1.00 | 3.88 | 1.16 | 4.14 | .006 |
| Demographic/Psycho-social | Distress – Depression | 4.20 | 0.97 | 4.25 | 0.93 | 4.02 | 1.13 | 4.00 | 1.04 | 4.15 | 1.09 | 2.23 | .083 |
| Demographic/Psycho-social | Self-reported health status | 2.42 | 1.01 | 2.42 | 0.98 | 2.45 | 1.02 | 2.42 | 1.10 | 2.28 | 1.00 | 1.00 | .393 |
| Benefits/Barriers | Health literacy | 4.43 | 1.04 | 4.54 | 0.94 | 4.23 | 1.25 | 3.99 | 1.27 | 4.26 | 1.12 | 13.12 | < .001 |
| Benefits/Barriers | Self-efficacy | 1.84 | 1.09 | 1.71 | 0.96 | 2.18 | 1.29 | 2.26 | 1.44 | 1.95 | 1.10 | 10.28 | < .001 |
| n | % | n | % | n | % | n | % | n | % | Wald χ2 | p | ||
| Benefits/Barriers | Ever heard of the cancer center | 987 | 82.5 | 763 | 86.0 | 55 | 70.5 | 131 | 70.8 | 31 | 79.5 | 17.80 | < .001 |
| Benefits/Barriers | Have a place they typically go for care | 959 | 80.2 | 730 | 82.3 | 60 | 76.9 | 136 | 73.5 | 27 | 69.2 | 5.63 | .131 |
| M | SD | M | SD | M | SD | M | SD | M | SD | F | p | ||
| Susceptibility/Severity | Beliefs about cancer | 2.38 | 0.50 | 2.37 | 0.49 | 2.42 | 0.55 | 2.41 | 0.50 | 2.29 | 0.45 | 1.28 | .282 |
| Susceptibility/Severity | Perceived absolute risk of cancer | 50.40 | 23.60 | 52.01 | 22.44 | 42.94 | 25.74 | 46.39 | 26.35 | 48.18 | 27.09 | 3.45 | .016 |
| Susceptibility/Severity | Perceived age-relative risk of cancer | 2.85 | 0.91 | 2.91 | 0.88 | 2.69 | 1.05 | 2.61 | 0.96 | 2.82 | 1.00 | 5.23 | .001 |
| Health behavior | Days of exercise per week | 3.88 | 2.26 | 3.97 | 2.30 | 3.97 | 2.23 | 3.49 | 2.12 | 3.56 | 2.15 | 1.64 | .178 |
| n | % | n | % | n | % | n | % | n | % | Wald χ2 | p | ||
| Health behavior | Smoked ≥ 100 cigarettes in entire life | 556 | 46.5 | 454 | 51.2 | 28 | 35.9 | 60 | 32.4 | 13 | 33.3 | 19.43 | < .001 |
| Health behavior | Ever had blood test for hepatitis C | 423 | 35.4 | 297 | 33.5 | 28 | 35.9 | 73 | 39.5 | 23 | 59.0 | 12.39 | .006 |
| Health behavior | Recommended to get HPV vaccine (self or relative in last 12 months)a | 133 | 11.1 | 80 | 9.0 | 9 | 11.5 | 35 | 18.9 | 9 | 23.1 | 10.87 | .012 |
| Health behavior | Ever recommended colorectal cancer screeninga | 535 | 44.7 | 425 | 47.9 | 27 | 34.6 | 60 | 32.4 | 19 | 48.7 | 6.35 | .096 |
| All femalesb (N = 731) |
WNH females (n = 554) |
BNH females (n = 41) |
H/L females (n = 115) |
ONH females (n = 17) |
Group differences | ||||||||
|
|
|
|
|
|
|
||||||||
| n | % | n | % | n | % | n | % | n | % | Wald χ2 | p | ||
| Health behavior | Ever had mammograma | 493 | 67.4 | 391 | 70.6 | 28 | 68.3 | 61 | 53.0 | 10 | 58.8 | 6.75 | .080 |
| Health behavior | Ever had Pap test | 620 | 84.8 | 489 | 88.3 | 33 | 80.5 | 81 | 70.4 | 13 | 76.5 | 10.37 | .016 |
| Health behavior | Ever had HPV test | 253 | 34.6 | 172 | 31.0 | 19 | 46.3 | 56 | 48.7 | 5 | 29.4 | 13.69 | .003 |
Significant effects are bolded. All analyses controlled for annual household income and relationship status
WNH White non-Hispanic/Latinx, BNH Black non-Hispanic/Latinx, H/L Hispanic/Latinx of any race, ONH other non-Hispanic/Latinx and belonging to a race other than White or Black (e.g., American Indian, Asian)
Age was included as an additional control variable
Participants were asked about gender, not biological sex; all participants identifying as female were administered items about mammograms, Pap tests, and HPV (human papillomavirus) tests
Demographic and psychosocial factors
Significant differences were observed between racial/ethnic groups for community attributes (F(3, 1077) = 9.36, p < 0.001), such that WNHs reported better community attributes than BNHs (p = 0.037), H/Ls (p < 0.001), and ONHs (p = 0.016). Significant differences were also observed for everyday discrimination (F(3, 1069) = 9.23, p < 0.001); specifically, WNHs reported less everyday discrimination than BNHs (p < 0.001), H/Ls (p = 0.036), and ONHs (p = 0.004), and BNHs reported more everyday discrimination than H/Ls (p = 0.011). Anxiety symptoms also differed significantly by racial/ethnic group (F(3, 1066) = 4.14, p = 0.006), such that WNHs reported significantly more anxiety than BNHs (p = 0.011) and H/Ls (p = 0.020). There were no significant differences for depression symptoms or self-reported health status.
Susceptibility and severity
Significant differences were seen in perceived absolute (F(3, 1077) = 3.45, p = 0.016) and age-relative (F(3, 1077) = 5.23, p = 0.001) risk of cancer. Specifically, WNHs perceived higher absolute risk than BNHs (p = 0.026), and they perceived both higher absolute (p = 0.012) and age-relative (p < 0.001) risk than H/Ls. There were no significant racial/ethnic group differences in their beliefs about cancer being caused by behavior or lifestyle.
Benefits and barriers to preventive health behaviors
Racial/ethnic group was significantly associated with health literacy (F(3, 1077) = 13.12, p < 0.001). Specifically, WNHs (p < 0.001) and BNHs (p = 0.049) both reported needing more help when reading written materials from providers/clinics (worse health literacy) compared to H/Ls. Racial/ethnic group was also associated with self-efficacy (F(3, 1077) = 10.28, p < 0.001), such that WNHs reported feeling significantly more confident about their ability to get advice and information about their health compared to BNHs (p = 0.026) and H/Ls (p < 0.001). Significant differences were observed in whether the participant had ever heard of the cancer center (Wald χ2(3) = 17.80, p < 0.001); compared to WNHs, BNHs (B = −0.77, Odds Ratio [OR] = 0.46, p = 0.010) and H/Ls (B = −0.76, OR = 0.47, p < 0.001) were significantly less likely to have ever heard of the cancer center. There were no significant group differences in likelihood of having a place they typically go to for health care.
Screening and prevention behaviors
Racial/ethnic group was significantly associated with smoking at least 100 lifetime cigarettes (Wald χ2(3) = 19.43, p < 0.001), with H/Ls (B = −0.71, OR = 0.49, p < 0.001) and ONHs (B = −0.80, OR = 0.45, p = 0.029) being significantly less likely to have smoked at least 100 cigarettes in their lifetime compared to WNHs. Significant differences were also seen across racial/ethnic groups in other cancer prevention and screening behaviors, including receipt of a blood test for hepatitis C (Wald χ2(3) = 12.39, p = 0.006) and having a health care provider recommend an HPV vaccine for the participant or family member in the previous 12 months (Wald χ2(3) = 10.87, p < 0.012). Specifically, compared to WNHs, H/Ls and ONHs were significantly more likely to have received a blood test for hepatitis C (H/Ls: B = 0.42, OR = 1.52, p = 0.030; ONHs: B = 1.11, OR = 3.04, p = 0.004) and to have a provider recommend an HPV vaccine (H/Ls: B = 0.70, OR = 2.00, p = 0.004; ONHs: B = 0.86, OR = 2.36, p = 0.038).
Among female-identifying participants, significant differences were also seen in receipt of a Pap test (Wald χ2(3) = 10.37, p = 0.016) and HPV test (Wald χ2(3) = 13.69, p = 0.003); compared to WNHs, H/Ls were less likely to have ever received a Pap test (B = −0.92, OR = 0.40, p = 0.002). but more likely to have ever received an HPV test (B = 0.85, OR = 2.35, p < 0.001).
No significant differences were seen across groups in days of exercise per week, likelihood of having a health care provider recommend colorectal cancer screening, or likelihood of female-identifying participants ever receiving a mammogram.
Discussion
Cancer health disparities are well-documented in the United States broadly but must be understood within local contexts to best serve local communities. This is especially true for NCI-Designated Comprehensive Cancer Centers that are tasked with addressing their catchment areas’ cancer burden and needs through research and community outreach and education. Using the HBM as a theoretical framework, we characterized racial/ethnic data from one cancer center’s Community Health Needs Assessment to better understand the population this cancer center serves and the needs of its catchment area.
In our study, similar to other work [25, 26], racial/ethnic minorities reported worse community attributes and more everyday discrimination. These factors are important to consider in terms of cancer prevention in that safe environments can promote cancer risk-reducing health behavior, such as exercise [27, 28]. Our findings suggest that physical infrastructure might be important to consider in improving health behaviors and associated health outcomes for REMs. This aligns with our catchment area community-driven priorities of improving access to care, including transportation and access to facilities that can address physical inactivity and obesity [29]. Further, Minority Stress Theory [30, 31] suggests that the cumulative stress of discrimination can increase health risk, over and above risk due to systemic and structural causes. For example, the need for vigilance against discrimination can decrease an individual’s an ability to self-regulate and impair self-control; as such, these individuals may have fewer resources to make healthy choices [30, 32], including engaging in cancer screening [33]. However, other research indicates that those with a strong minority-based identity are perhaps better-equipped to manage minority stressors, invalidate stereotypes, and dismiss or address perceived or actual discrimination [34, 35]. As such, it may be beneficial for health care and health-based community organizations to engage in outreach that aligns with the specific minority group identity in the communities they serve. Similarly, population science-based research must consider the health beliefs and cultural beliefs of the population served by the center.
The primary benefits and barriers that we assessed referred to participants’ ability to obtain and assess health information. An individual’s ability to understand and integrate health information is an important factor when it comes to engaging in preventative health behaviors [36] and thus improving health outcomes [37]. A lack of understanding can also translate into misperceptions about cancer and its treatment [38]. Although, in our study, there were racial/ethnic differences in needing help with written medical materials, all groups reported relatively high levels of literacy and confidence in their ability to get advice and information regarding their health.
Despite this confidence in obtaining information, BNHs and H/Ls had 54% and 53% lower odds, respectively, to have ever heard about the cancer center compared to WHNs; this suggests that some racial/ethnic minority groups may be more familiar with community-based cancer organizations and potentially more likely to seek and receive care there. While excellent care can be provided in these settings, NCI-designated Comprehensive Cancer Centers often offer specialized cancer care and clinical trials based on the latest scientific advances in our understanding of cancer [39]. Racial and ethnic minorities are under-represented in clinical research [7] which can further perpetuate disparities. One potential area for growth at our cancer center is fostering partnerships between our cancer center and community-based health care systems. In other settings where NCI-designated Comprehensive Cancer Centers have partnered with community health care organizations, traditionally underserved racial and ethnic groups can gain better access to biomedical research [40], which both offers opportunities for novel treatment and ensures more equitable representation in basic science.
BNHs and H/Ls in this study perceived a lower absolute risk of cancer compared to WNHs, and while WNHs represent slightly higher rates of new cancer cases per 100,000 people, cancer death rates are highest among Black males and females [41]. Our BNH and H/L participants may have felt at lower risk based on their self-reported relatively higher rates of risk-reducing health behaviors, such as testing rates for Hepatitis C and HPV, and lower lifetime smoking rates. However, it appears that WNHs were much more likely to have been recommended or to undergo some cancer screening like Pap tests. Lack of preventative health screenings can lead to cancers being detected at later stages with worse outcomes [42]. These discrepancies highlight ways in which our cancer center can attend to its catchment area, perhaps by targeting research and outreach to racial and ethnic minority women for Pap tests and WNHs for smoking cessation programs. Cervical cancer and lung cancer both have higher incidence rates in our catchment area than the state and nation; our findings emphasize the importance of providing access to early detection and prevention tools to BNHs and H/Ls, both growing groups in our area [29].
Limitations and future directions
Several limitations should be considered in the interpretation of our findings. First, this was a secondary analysis of cross-sectional data; therefore, results should be interpreted in context of the weaknesses inherent with this study design. Second, several diverse racial subgroups were collapsed into groups—those who identified as Hispanic/Latinx were combined into a single H/L group regardless of race, and all participants who were not White, Black, or Hispanic/Latinx were combined into a single “Other” group. There were not enough participants belonging to these specific racial subgroups (e.g., American Indian vs. Asian) to conduct more fine-grained analyses examining differences among them, though there are likely important subgroup differences in barriers and facilitators to engaging in health behaviors. Future assessments will target larger samples of racial/ethnic groups to begin to explore differences. Using an online survey panel facilitated obtaining a representative sample; however, this prevented calculation of response rates. The survey was only offered in English and Spanish, the two primary languages spoken in our catchment area, but this may have excluded those who spoke other languages. Finally, measures used to assess HBM constructs were limited in length to reduce participant burden.
Despite these limitations, information from this Community Health Needs Assessment served to facilitate discussions with key stakeholders within and outside the cancer center to guide the development of shared priorities for the center, including research and outreach efforts.
Conclusions
Results from our triennial Community Health Needs Assessment survey identify areas in which certain racial and ethnic minority groups differ significantly from WNHs in ways that may affect cancer prevention, diagnosis, treatment, and survival. Our cancer center is now tasked with working to mitigate these disparities in our catchment area through targeted and culturally tailored research and outreach efforts. While cancer health disparities are a problem on a national level, local data is needed in order to address the specific context and concerns of populations that are served by NCI-designated Comprehensive Cancer Centers.
Acknowledgments
Funds from the National Cancer Institute supported this project (P30CA076292, PI: J. Cleveland; T32CA090314, PIs: T. H. Brandon & S. T. Vadaparampil). We would like to thank the Moffitt Diversity Team (Cathy Lee), individuals involved in developing the methodology and finalizing the questionnaire (including Dr. B. Lee Green, Cathy Grant, Chantel Griffin Stampfer, Dr. Shelley Tworoger, Dr. Jenny Permuth, Dr. Clement Gwede, Dr. Travis Gerke, and Dr. Margaret Byrne), as well as catchment area participants who completed questionnaires.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Ethical approval This study was determined by Moffitt Cancer Centers’ Institutional Review Board not to be human subjects research and was therefore exempt from review.
Data availability
The data that support the findings of this study are available upon reasonable request from the senior author, Dr. Susan T. Vadaparampil (susan.vadaparampil@moffitt.org).
References
- 1.National Cancer Institute (2020) NCI Center to Reduce Cancer Health Disparities (CRCHD). https://www.cancer.gov/about-nci/organization/crchd. Accessed 6 Jan 2020.
- 2.Vaccarella S, Lortet-Tieulent J, Saracci R, Fidler MM, Conway DI, Vilahur N et al. (2018) Reducing social inequalities in cancer: setting priorities for research. CA: A Cancer J Clin 68(5):324–326 [DOI] [PubMed] [Google Scholar]
- 3.Singh GK, Jemal A (2017) Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health 2017:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray TF, Cudjoe J, Murphy J, Thorpe RJ, Wenzel J, Han H-R (2017) Disparities in cancer screening practices among minority and underrepresented populations. Semin Oncol Nurs 33(2):184–198. 10.1016/j.soncn.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 5.Valdovinos C, Penedo FJ, Isasi CR, Jung M, Kaplan RC, Giacinto RE et al. (2016) Perceived discrimination and cancer screening behaviors in US hispanics: the hispanic community health study/study of latinos sociocultural ancillary study. Cancer Causes Control 27(1):27–37. 10.1007/s10552-015-0679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moses KA, Orom H, Brasel A, Gaddy J, Underwood W (2017) Racial/Ethnic disparity in treatment for prostate cancer: does cancer severity matter? Urology 99:76–83. 10.1016/j.urology.2016.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S (2016) Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 23(4):327–337. 10.1177/107327481602300404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kish JK, Yu M, Percy-Laurry A, Altekruse SF (2014) Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in surveillance, epidemiology, and end results (SEER) registries. JNCI Monographs 2014(49):236–243. 10.1093/jncimonographs/lgu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL (2018) Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 36(1):25–33. 10.1200/JCO.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apenteng BA, Hansen AR, Opoku ST, Mase WA (2017) Racial disparities in emotional distress among cancer survivors: insights from the health information national trends survey (HINTS). J Cancer Educ 32(3):556–565. 10.1007/s13187-016-0984-7 [DOI] [PubMed] [Google Scholar]
- 11.Mollica MA, Lines LM, Halpern MT, Ramirez E, Schussler N, Urato M et al. (2017) Patient experiences of cancer care: scoping review, future directions, and introduction of a new data resource: surveillance epidemiology and end results-consumer assessment of healthcare providers and systems (SEER-CAHPS). Patient Exp J 4(1):103–121 [Google Scholar]
- 12.Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR et al. (2017) Charting the future of cancer health disparities research: a position statement from the american association for cancer research, the american cancer society, the american society of clinical oncology, and the national cancer institute. Can Res 77(17):4548–4555. 10.1158/0008-5472.Can-17-0623 [DOI] [PubMed] [Google Scholar]
- 13.Finney Rutten LJ, Wilson PM, Jacobson DJ, Agunwamba AA, Radecki Breitkopf C, Jacobson RM et al. (2017) A population-based study of sociodemographic and geographic variation in HPV vaccination. Cancer Epidemiol Biomark Prev 26(4):533–540. 10.1158/1055-9965.Epi-16-0877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz Z, Zhang X, Richards TB, Nadel M, Peipins LA, Holt J (2018) Multilevel small-area estimation of colorectal cancer screening in the United States. Cancer Epidemiol Biomark Prev 27(3):245–253. 10.1158/1055-9965.Epi-17-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein A, Gentzkow M, Williams H (2016) Sources of geographic variation in health care: evidence from patient migration*. Q J Econ 131(4):1681–1726. 10.1093/qje/qjw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janz NK, Becker MH (1984) The health belief model: a decade later. Health Educ Q 11(1):1–47. 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- 17.Davis JL, Buchanan KL, Green BL (2013) Racial/ethnic differences in cancer prevention beliefs: applying the health belief model framework. American J Health Promotion: AJHP 27(6):384–389. 10.4278/ajhp.120113-QUAN-15 [DOI] [PubMed] [Google Scholar]
- 18.Johnson CE, Mues KE, Mayne SL, Kiblawi AN (2008) Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the health belief model. J Low Genit Tract Dis 12(3):232–241. 10.1097/LGT.0b013e31815d8d88 [DOI] [PubMed] [Google Scholar]
- 19.Rollins L, Sy A, Crowell N, Rivers D, Miller A, Cooper P et al. (2018) Learning and action in community health: using the health belief model to assess and educate african american community residents about participation in clinical research. Int J Environ Res Public Health 15(9):1862. 10.3390/ijerph15091862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JE, Zane N (2016) Help-seeking intentions among Asian American and White American students in psychological distress: application of the health belief model. Cultur Divers Ethnic Minor Psychol 22(3):311–321. 10.1037/cdp0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai CG, Hiatt RA (2017) The population burden of cancer: research driven by the catchment area of a cancer center. Epidemiol Rev 39(1):108–122. 10.1093/epirev/mxx001 [DOI] [PubMed] [Google Scholar]
- 22.Grant CG, Ramos R, Davis JL, Lee GB (2015) Community health needs assessment: a pathway to the future and a vision for leaders. Health Care Manag (Frederick) 34(2):147–156. 10.1097/HCM.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 23.Blake KD, Ciolino HP, Croyle RT (2019) Population health assessment in NCI-designated cancer center catchment areas. Cancer Epidemiol Biomarkers Prev 28(3):428–430. 10.1158/1055-9965.Epi-18-0811 [DOI] [PubMed] [Google Scholar]
- 24.Champion VL, Skinner CS (2008) The health belief model. In: Glanz K, Rimer BK, Viswanath K (eds) Health behavior and health education, 4th edn. Jossey-Bass, San Francisco, CA, pp 45–66 [Google Scholar]
- 25.Gong F, Xu J, Takeuchi DT (2017) Racial and ethnic differences in perceptions of everyday discrimination. Sociol Race Ethnicity 3(4):506–521 [Google Scholar]
- 26.Calloway EE, Parks CA, Bowen DJ, Yaroch AL (2019) Environmental, social, and economic factors related to the intersection of food security, dietary quality, and obesity: an introduction to a special issue of the translational behavioral medicine journal. Transl Behav Med 9(5):823–826. 10.1093/tbm/ibz097 [DOI] [PubMed] [Google Scholar]
- 27.Richardson AS, Ghosh-Dastidar M, Collins RL, Hunter GP, Troxel WM, Colabianchi N et al. (2020) Improved street walkability, incivilities, and esthetics are associated with greater park use in two low-income neighborhoods. J Urban Health 97(2):204–212. 10.1007/s11524-019-00416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd M, Adams MA, Kurka J, Conway TL, Cain KL, Buman MP et al. (2016) GIS-measured walkability, transit, and recreation environments in relation to older adults physical activity: a latent profile analysis. Prev Med 93:57–63. 10.1016/j.ypmed.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Office of Community Outreach Engagement and Equity. Moffitt Cancer Center Catchment Area Profile. October, 2020. Retrieved from https://moffitt.org/research-science/outreach/community-outreach-engagement-and-equity/catchment-area-cancer-data/
- 30.Pascoe EA, Smart RL (2009) Perceived discrimination and health: a meta-analytic review. Psychol Bull 135(4):531–554. 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark R, Anderson NB, Clark VR, Williams DR (1999) Racism as a stressor for African Americans. A Biopsychosocial Model Am Psychol 54(10):805–816. 10.1037//0003-066x.54.10.805 [DOI] [PubMed] [Google Scholar]
- 32.Inzlicht M, McKay L, Aronson J (2006) Stigma as ego depletion: how being the target of prejudice affects self-control. Psychol Sci 17(3):262–269. 10.1111/j.1467-9280.2006.01695.x [DOI] [PubMed] [Google Scholar]
- 33.Myers RE (2003) Self-regulation and decision-making about cancer screening. In: Cameron LD, Leventhal H (eds) The self-regulation of health and illness behaviour. Routledge, New York, NY, pp 297–313 [Google Scholar]
- 34.Cobb CL, Meca A, Branscombe NR, Schwartz SJ, Xie D, Zea MC et al. (2019) Perceived discrimination and well-being among unauthorized Hispanic immigrants: the moderating role of ethnic/racial group identity centrality. Cultur Divers Ethnic Minor Psychol 25(2):280–287. 10.1037/cdp0000227 [DOI] [PubMed] [Google Scholar]
- 35.Forsyth J, Carter RT (2012) The relationship between racial identity status attitudes, racism-related coping, and mental health among Black Americans. Cultur Divers Ethnic Minor Psychol 18(2):128–140. 10.1037/a0027660 [DOI] [PubMed] [Google Scholar]
- 36.White S, Chen J, Atchison R (2008) Relationship of preventive health practices and health literacy: a national study. Am J Health Behav 32(3):227–242 [DOI] [PubMed] [Google Scholar]
- 37.Paasche-Orlow MK, Wolf MS (2010) Promoting health literacy research to reduce health disparities. J Health Commun 15(sup2):34–41. 10.1080/10810730.2010.499994 [DOI] [PubMed] [Google Scholar]
- 38.Gansler T, Henley SJ, Stein K, Nehl EJ, Smigal C, Slaughter E (2005) Sociodemographic determinants of cancer treatment health literacy. Cancer 104(3):653–660 [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute (2019) NCI-designated cancer centers. Retrieved from https://www.cancer.gov/research/infrastructure/cancer-centers. Accessed 20 May 2020
- 40.Barrett NJ, Rodriguez EM, Iachan R, Hyslop T, Ingraham KL, Le GM et al. (2020) Factors associated with biomedical research participation within community-based samples across 3 national cancer institute-designated cancer centers. Cancer 126(5):1077–1089. 10.1002/cncr.32487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SEER Cancer Statistics Factsheets: Common Cancer Sites. National Cancer Institute, Bethesda, MD. 2020. https://seer.cancer.gov/statfacts/html/disparities.html. Accessed 20 May 2020. [Google Scholar]
- 42.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D et al. (2019) Cancer screening in the United States, 2019: a review of current American cancer society guidelines and current issues in cancer screening. CA: A Cancer J Clin 69(3):184–210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the senior author, Dr. Susan T. Vadaparampil (susan.vadaparampil@moffitt.org).


