FIG 1.
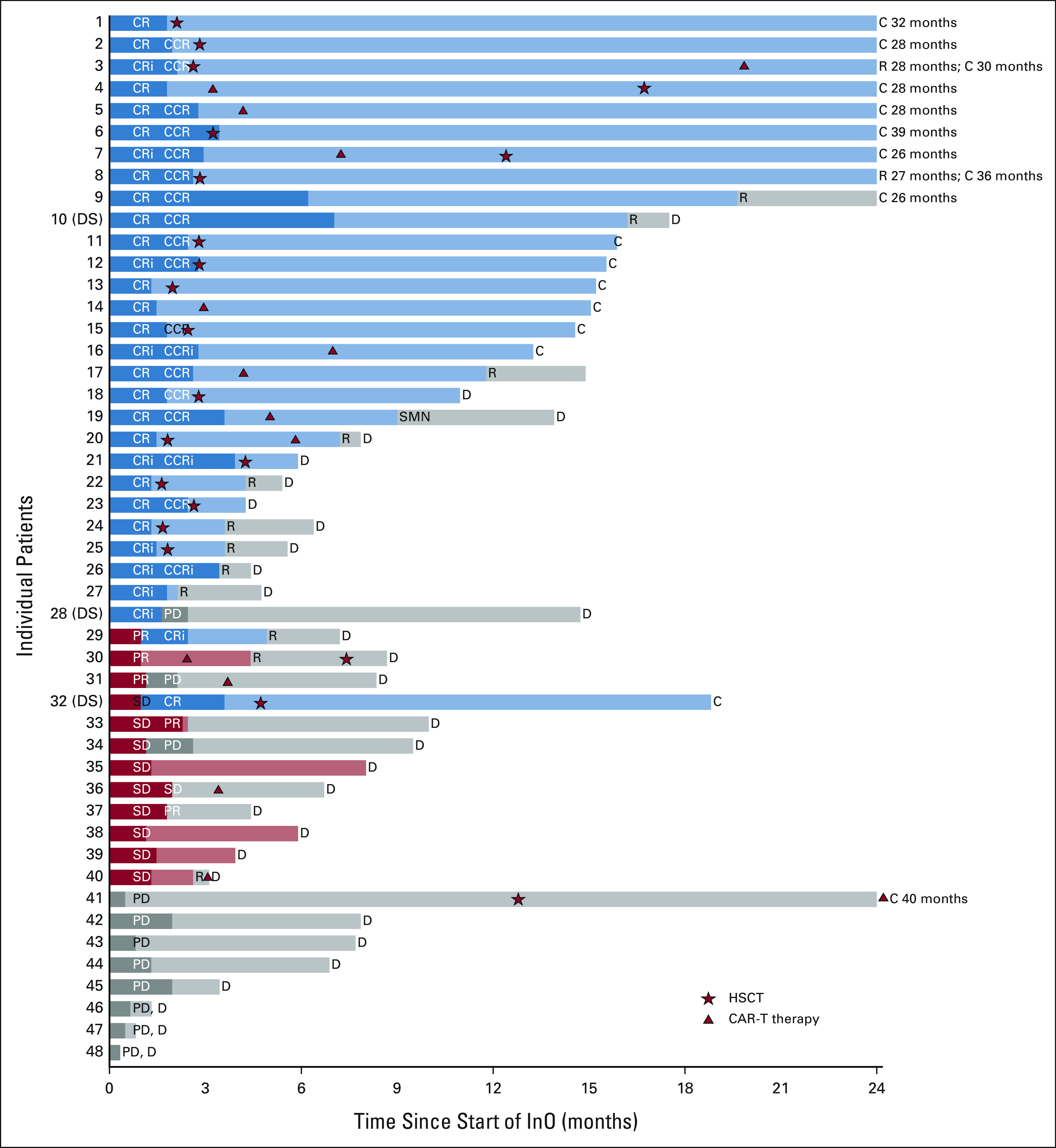
Swimmer's plot depicting individual patient response, subsequent therapy, and outcomes for 48 patients. Data are censored as of December 31, 2020. Patients with Down syndrome are denoted as DS. Dark bars indicate InO given on study. Initial response to cycle 1 is indicated by CR, CRi, PR, SD, or PD. For patients who received cycle 2, response is indicated as CCR or CCRi if previously achieved CR or CRi with cycle 1. Duration of initial response is indicated by color (CR or CRi = blue; PR or SD = red, PD = gray). HSCT is indicated by star, and CAR T-cell therapy is indicated by triangle. Patient 9 received six cycles of therapy on study and continued to receive commercial InO for an additional year before relapse. Patient 19 with KMT2A rearrangement received CD19 CAR T-cell therapy following three cycles of InO and subsequently developed lineage switch to acute myeloid leukemia (denoted as SMN). C, censored; CAR, chimeric antigen receptor; CCR, continuous complete response; CCRi, continuous complete response with incomplete count recovery; CR, complete response with or without count recovery; CRi, complete response with incomplete count recovery; D, death; DS, Down syndrome; HSCT, hematopoietic stem-cell transplantation; InO, inotuzumab ozogamicin; PD, progressive disease; PR, partial response; R, relapse; SD, stable disease; SMN, second malignant neoplasm.
