FIG 3.
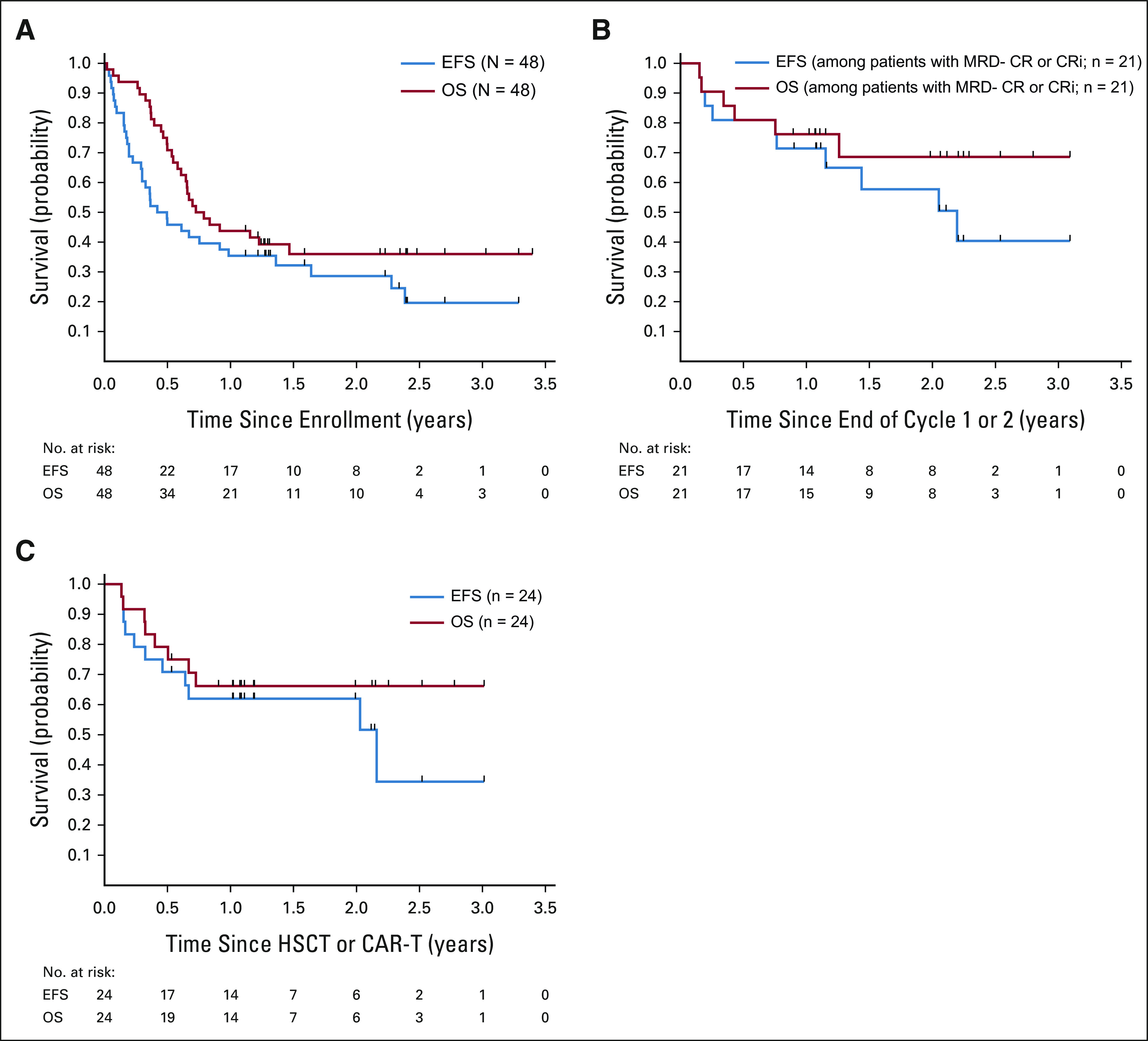
Outcomes after InO monotherapy. (A) EFS and OS of the 48 patients treated on study. The estimated EFS rate was 28.6% (95% CI, 15.9 to 42.8) at 2 years and 19.6% (95% CI, 7.9 to 35.1) at 3 years. The estimated OS rate was 36.0% (95% CI, 22.3 to 49.9) at 2 years or 3 years. (B) EFS and OS of patients with morphologic CR or CRi and MRD < 0.01% by flow cytometry with one or two cycles of treatment. Among patients with best response of CR or CRi and MRD < 0.01%, EFS was 71.4% (95% CI, 47.2 to 86) at 1 year and 57.7% (95% CI, 31.9 to 76.8) at 2 year, and the 2-year OS rate was 68.6% (95% CI, 41.8 to 84.9). (C) EFS and OS of patients who received HSCT or CART without other bridging therapy following a best response of CR or CRi after up to two cycles of InO. Of the 30 patients who achieved CR or CRi after up to two cycles of InO, four patients had an event at the end of InO therapy or shortly after being off protocol, two patients did not have HSCT or CART within 6 months without other therapy, and the remaining 24 patients had HSCT or CART following CR or CRi. Of the 24 patients who received HSCT or CART following CR or CRi, 2-year EFS and OS rates were 62.0%% (95% CI, 39.6 to 78.1) and 66.2% (95% CI, 43.6 to 81.5), respectively. CART, chimeric antigen receptor T-cell therapy; CR, complete response; CRi, complete response with incomplete count recovery; EFS, event-free survival; HSCT, hematopoietic stem-cell transplantation; InO, inotuzumab ozogamicin; MRD, minimal residual disease; OS, overall survival.
