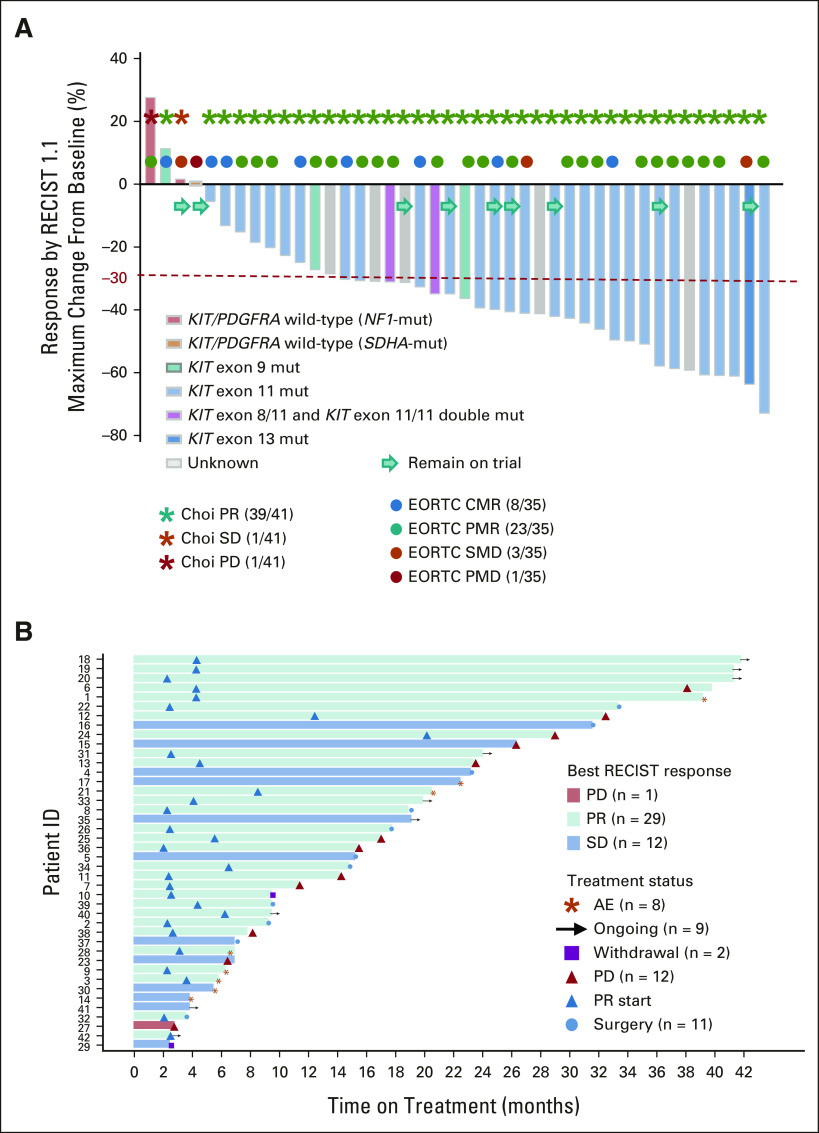
FIG 2.
Response rates (RECIST1.1, Choi, and EORTC) and duration of response. (A) Best objective responses by RECIST1.1 (n = 42), Choi responses (n = 41) around 8 weeks (end of cycle 2, first post-treatment scan), and EORTC responses (n = 35) by PET at 4 weeks (end of cycle 1) on combination imatinib and binimetinib treatment. The best RECIST1.1 responses are shown as % of change from baseline for patients who received the combination of imatinib and binimetinib and with at least one postbaseline scan. The known associated primary driver mutations in KIT, PDGFRA, and others are shown. The best ORR was 69.0% (29 of 42 confirmed PR), two-sided 95% CI: 52.9 to 82.4. (B) Duration of the response. AE, adverse events; CMR, complete metabolic response; EORTC, European Organisation for Research and Treatment of Cancer; mut, mutant; ORR, objective response rate; PD, progression of disease; PET, positron emission tomography; PMD, progressive metabolic disease; PMR, partial metabolic response; PR, partial response; SD, stable disease; SMD, stable metabolic response.