Figure 1.
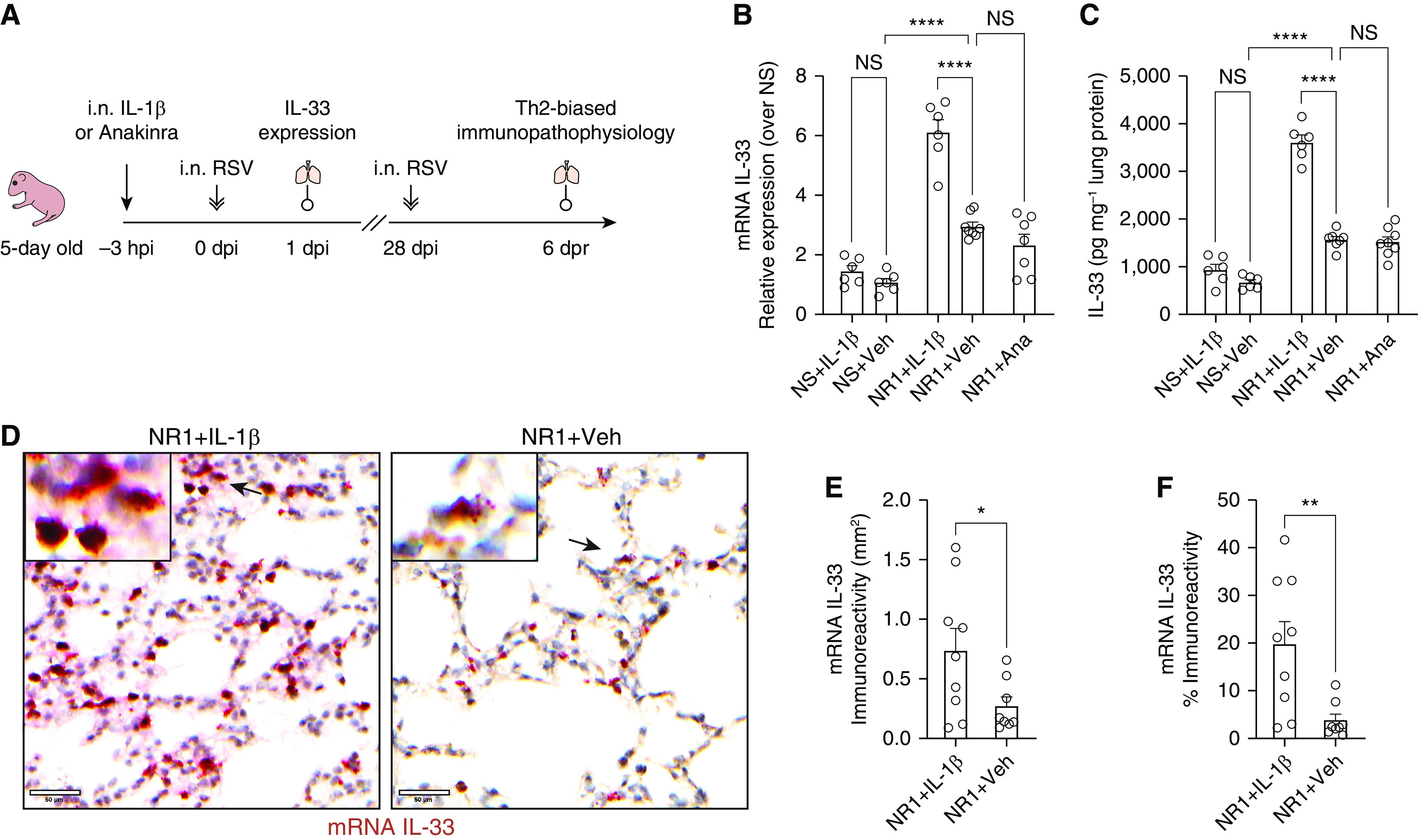
Intranasal treatment with IL-1β before respiratory syncytial virus (RSV) infection significantly boosted the expression of RSV-induced IL-33 at 1 day after infection (dpi) in neonatal mice. (A) Experiment design scheme. Five-day-old pups received either 2.5 ng of recombinant IL-1β/g body weight (Biolegend) or 50 μg anakinra (IL-1β antagonist)/g body weight delivered intranasally (i.n.) 3 hours before i.n. RSV infection. The expression of pulmonary IL-33 was examined at 1 dpi (hereafter referred to as NR1 + IL-1β or NR1 + Ana, respectively). A cohort of mice received vehicle (0.1% BSA in saline) i.n. and served as the control group (NR1 + Veh). We reinfected mice with RSV 4 weeks after initial infection. At 6 days after reinfection (dpr), we profiled pulmonary cell subsets and evaluated the animals’ lung function. (B and C) Bar graphs representing the expression of IL-33 mRNA (B) and protein (C). (D) Representative IL-33 in situ hybridization (ISH) sections of lungs from three independent RSV-infected neonatal mice. IL-33 mRNA was stained as dark red dots indicated by black arrows within the magnified inset (top left). Scale bars, 50 μm. (E and F) Quantification of IL-33 mRNA–positive cells per unit area of lungs. ISH sections were digitized to 20× whole slide images and presented as the staining area (E) and proportions of the whole slide area (F). *P < 0.05, **P < 0.01, and ****P < 0.0001; two-tailed Welch’s t-tests and one-way ANOVA with a Sidak multiple comparisons compared between two or more than two indicated groups, respectively. Ana = anakinra; hpi = hours post-injection; NS = not significant; Veh = vehicle; Th2 = T-helper cell type 2.
