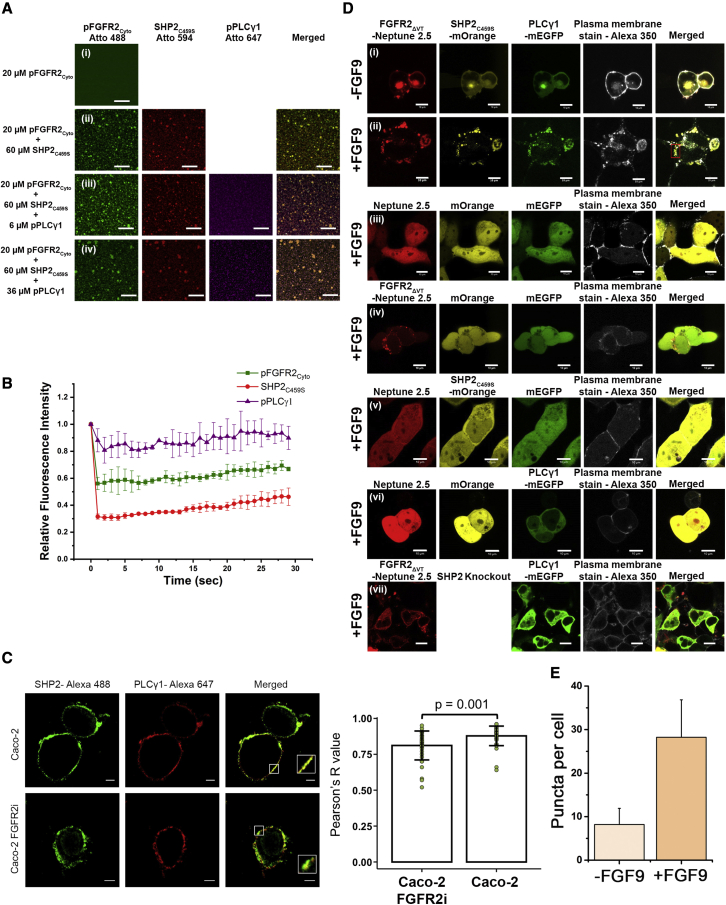
Figure 3.
The formation of LLPS pFGFR2-SHP2C459S-pPLCγ1 condensates on supported lipid bilayers and plasma membranes
(A) pFGFR2Cyto-SHP2C459S-pPLCγ1 condensates on supported lipid bilayers. (i) Confocal images of homogeneously distributed pFGFR2Cyto Atto-488 (20 μM, 6xHis tagged) on membrane bilayers, (ii) pFGFR2Cyto Atto-488 gradually clustered upon the addition of SHP2C459S Atto-594 (60 μM, untagged), and (iii) pPLCγ1 Atto-647 (6 μM, untagged), followed by (iv) additional 36 μM of untagged pPLCγ1 Atto-647. Scale bars, 10 μm.
(B) FRAP analysis showing the dynamic nature of pFGFR2Cyto-SHP2C459S-pPLCγ1 condensates on supported lipid bilayers as all pFGFR2Cyto, SHP2C459S, and pPLCγ1 exchanged with their counterparts in the dilute phase. Data are presented as mean ± SD, n = 2 experiments.
(C) Immunofluorescence staining images showing colocalized SHP2-Alexa 488 and PLCγ1-Alexa 647 droplet formation on plasma membrane in FGF9-stimulated (10 ng/ml, 15 min) Caco-2 cells and Caco-2 FGFR2i cells. Inset image: magnification of regions shown to exemplify endogenous SHP2-PLCγ1 clusters on membranes. Graph (right of image): statistical analysis of droplet formation in parental Caco-2 cells and Caco-2 FGFR2i cells. Only the SHP2-Alexa 488 and PLCγ1-Alexa 647 colocalized droplets were counted. Knocking down FGFR2 reduces SHP2-Alexa 488 and PLCγ1-Alexa 647 colocalized droplets. Degree of colocalization of endogenous PLCγ1 and SHP2 determined by Pearson's R value. Sample numbers = 37 (wild type) or 38 (FGFR2i) from 2 independent experiments.
(D) Live cell images showing FGFR2ΔVT-SHP2C459S-PLCγ1 LLPS droplet formation on plasma membrane upon FGFR2ΔVT expression and activation in HEK293T SHP2 KO cells. FGFR2ΔVT, SHP2C459, and PLCγ1 were tagged with Neptune 2.5, mOrange, and mEGFP, respectively. Alexa 350-conjugated wheat germ agglutinin was used to stain the plasma membrane. (i) Serum-starved (-FGF9) cells show a low level of FGFR2ΔVT-SHP2C459S-PLCγ1 droplets colocalized on membrane; this could have been due to protein recruitment by the basally activated FGFR2. Most SHP2 and PLCγ1 proteins are diffused in cytosol. (ii) FGF9-stimulation (+FGF9, 10 ng/ml for 15 min) led to the activation of FGFR2 and enhanced FGFR2ΔVT-SHP2C459S-PLCγ1 LLPS droplet formation on the plasma membrane (see also Video S1). (iii) Expression of fluorescent tags alone does not initiate the droplet formation. (iv) In the absence of SHP2C459S-mOrange and PLCγ1-mEGFP, activated FGFR2ΔVT still forms droplets on the membrane with other endogenous cellular proteins. (v) SHP2C459S-mOrange does not form droplets in the absence of FGFR2ΔVT. (vi) PLCγ1-mEGFP does not form droplets in the absence of FGFR2ΔVT. (vii) FGFR2ΔVT cannot recruit active PLCγ1-mEGFP to the membrane in the absence of SHP2 expression, resulting in the random diffusion of PLCγ1-mEGFP.
(E) Statistical analysis of FGFR2ΔVT-SHP2C459S-PLCγ1 LLPS droplet formation in the absence (light peach) or presence (dark peach) of FGF9 stimulation. Sample numbers = 40 per condition from 3 independent experiments. Data are presented as mean ± SD.