Figure 7.
Phase transition of FGFR2-SHP2-PLCγ1 upregulates downstream signaling
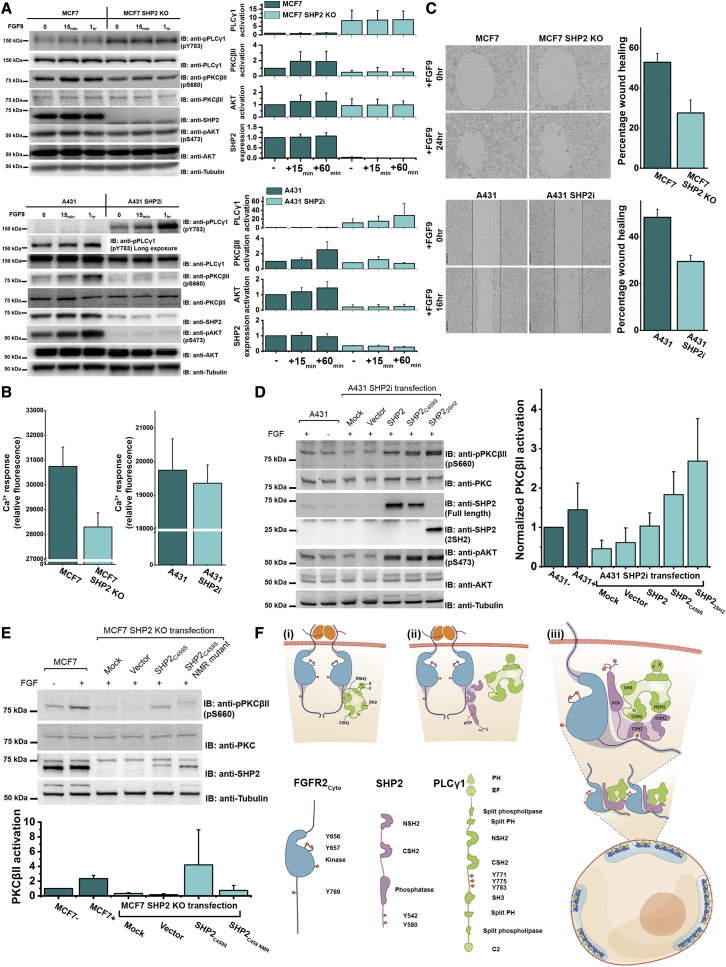
(A) (Left) Depletion of SHP2 upregulates PLCγ1 through phosphorylation of Y783 but downregulates its downstream effectors (shown by reduced phosphorylation of PKCβII-S660 and AKT-S473) in FGF9-stimulated (10 ng/ml) MCF7 cells and A431 cells. (Right) Densitometry analysis of SHP2 expression and the activation levels of various signaling proteins (dark green: parental cells; light green: SHP2 depletion cells). MCF7 cells: n = 3; A431 cells: n = 2. Data are presented as mean ± SD. Replicate data are shown in Data S3A.
(B) Inhibition of calcium response in MCF7 SHP2 KO cells (sample size = 8) and A431 SHP2i cells (sample size = 16) (light cyan) upon FGF9 stimulation (10 ng/ml) for 1 h compared with the parental cells (dark cyan).
(C) (Left) Cell motility is reduced in the SHP2 depletion cells upon FGF9 stimulation (10 ng/ml) (MCF7 SHP2 KO cells and A431 SHP2i cells) compared with control the parental cells. (Right) Graphical representation of percentage recovery; (dark cyan) parental cells, (light cyan) SHP2 depletion cells (n = 3).
(D) (Left) Knockin SHP2 constructs restore PKCβII activity upon FGF9 stimulation (10 ng/ml) in A431 SHP2i cells. Knockin wild-type SHP2 has lower effect on restoring PKCβII activity, which could have been due to the rapid phospho-turnover mediated by overexpressed SHP2. Both SHP2C459S and SHP22SH2 greatly increase PKCβII activity, indicating that the phosphatase activity is dispensable and only the tandem SH2 domains are required. (Right) Densitometry analysis of PKCβII activity upon knock in of various SHP2 constructs (dark cyan: parental cells; light cyan: SHP2 depletion cells), n = 3. Data are presented as mean ± SD. Replicate data are shown in Data S3B.
(E) (Top) Mutations of the PLCγ1 binding interface residues on SHP2 tandem SH2 domains (SHP2C459S NMR) abolish the ability of SHP2C459S to restore PKCβII activity in MCF7 SHP2 KO cells. (Bottom) Densitometry analysis of PKCβII activity upon knockin of various SHP2 constructs (dark cyan: parental cells; light cyan: SHP2 depleted cells), n = 3. Data are presented as mean ± SD. Replicate data are shown in Data S4C.
(F) Schematic diagrams of the domains of FGFR2Cyto, SHP2, and PLCγ1. (i) Upon ligand stimulation (orange), membrane-localized FGFR2 (blue) recruits the NSH2 domain of PLCγ1 (green) into pY769 on its C terminus. This results in the phosphorylation (pY783), activation dissociation from FGFR2 of PLCγ1. FGFR2 can also recruit SHP2 (purple) CSH2 domain into pY769 (ii). The FGFR2-SHP2 complex is then available to recruit the active PLCγ1 through the tandem SH2 domains of SHP2 and PLCγ1. The “secondary interaction” mediated by the SHP2 NSH2 domain and pYs on the FGFR2 kinase domain further provide the multivalency for the phase separation of the ternary complexes on cellular membrane (iii).