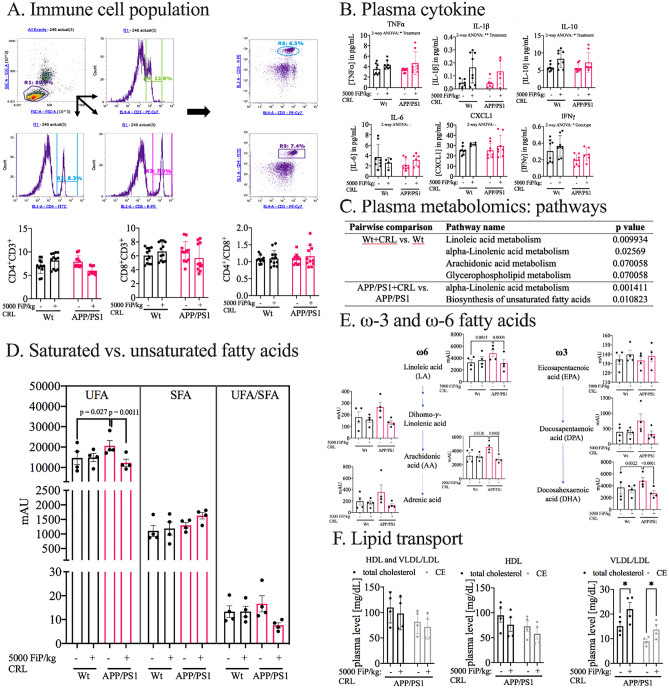
Figure 2.
Peripheral impact of CRL treatment. A Quantification of immune cell population by flow cytometry analysis. No difference was observed in CD4+CD3+, CD8+CD3+ and their ratio, respectively. B Peripheral inflammation measured by plasma cytokine and chemokine levels. No biologically-relevant significant changes could be determined. C Pathway analysis of untargeted plasma metabolomics data indicating significant changes in UFA and glycerophospholipid metabolism. D Investigation of subclasses of UFA, SFA and UFA/SFA revealed an UFA specific elevation in APP/PS1 mice and reduction post treatment. E ω-3 and ω-6 fatty acid levels measured in plasma. All subtypes showed significant or trending normalization of UFA level post CRL treatment in APP/PS1 mice. F HDL and VLDL/LDL cholesterol and cholesterol esters to analyze CRL’s effect on lipid transport. VLDL/LDL cholesterol and cholesterol ester fraction were both significantly increased. Significance was assessed by 2-way ANOVA and post-hoc Tukey correction. Significance: *p < 0.05, **p < 0.01, ***p < 0.001.