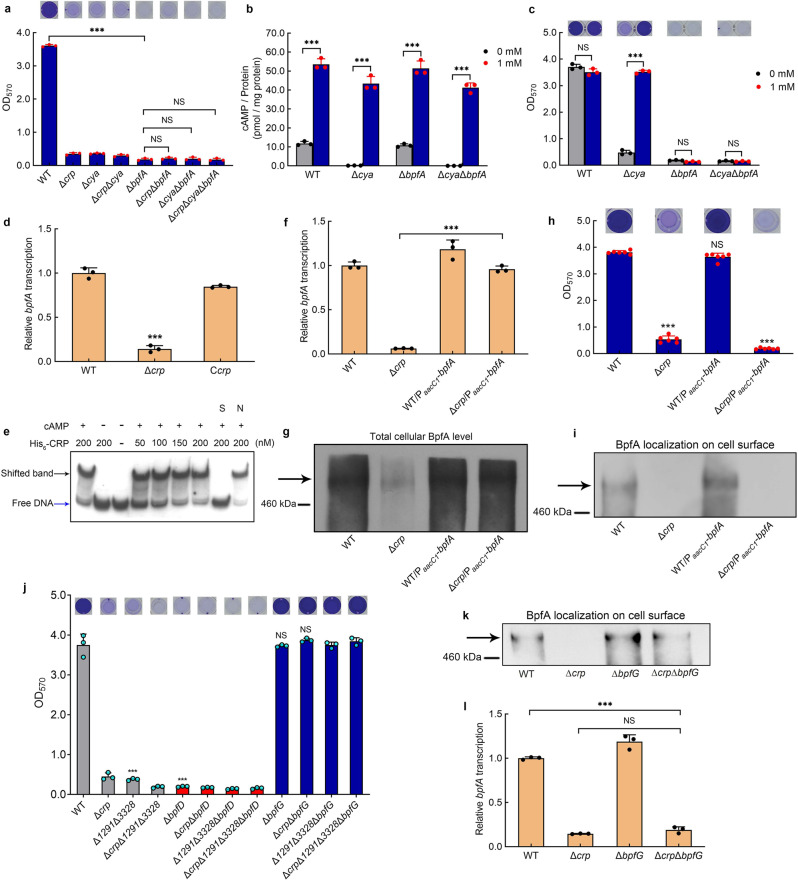
Fig. 3. cAMP-CRP maintains biofilm independently of its regulation of bpfA transcription.
a Biofilm biomass (n = 3 independent samples). b Intracellular cAMP concentration with adding 1 mM exogenous cAMP to the culture medium vs. control (n = 3 independent samples). c Biofilm biomass with adding 1 mM exogenous cAMP to the culture medium vs. control (n = 3 independent samples). d Transcriptional analysis of bpfA (n = 3 independent samples). e EMSA of cAMP-CRP binding to bpfA promoter (bpfA-pro). The His6-CRP (Lane–), labeled probe was incubated in the absence of His6-CRP. The concentrations of His6-CRP are shown above the figure. The binding specificity was confirmed by competitive assays with a 300-fold excess of unlabeled specific probe bpfA-pro (lane S) or unlabeled nonspecific competitor DNA (probe recA) (lane N). The cAMP (Lane –), labeled probe was incubated in the absence of cAMP. The cAMP (Lane + ), labeled probe was incubated in 1 μM cAMP. f The comparison of bpfA transcription between the native bpfA promoter and the constitutive promoter PaacC1 (n = 3 independent samples). g Western blotting detection of the total content of cellular BpfA protein. h Biofilm biomass (n = 6 independent samples). i Western blotting detection of BpfA localization on the cell surface. j Biofilm biomass (n = 3 independent samples). k Western blotting detection of BpfA localization on the cell surface. l Transcriptional analysis of bpfA (n = 3 independent samples). All strains used in (a–d, f–l) were cultured for 30 h in the biofilm state. Insets in (a, c, h, j) are the biofilm pictures of crystal violet dyeing. Data in (a–d, f, h, j, l) are shown as the mean ± SD. Two-sided Student’s t test was used in (a–d, f, h, j, l) to analyze the statistical significance (NS: No significance. ***p < 0.001). Source data are provided as a Source Data file.