Fig. 6. AVR-Pia associates with RGA5_ΔHMA but not AVR-PikD.
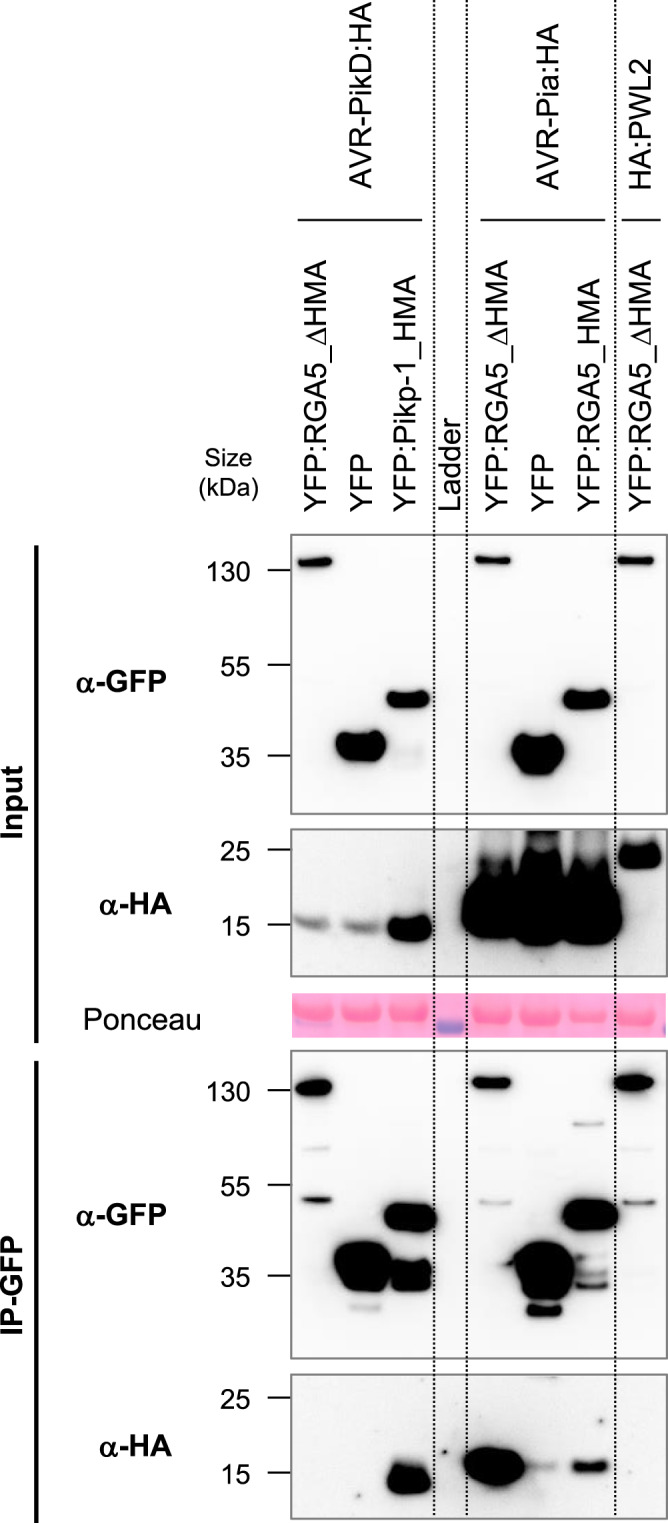
RGA5 deleted of its C-terminal domain (RGA5_ΔHMA, residues 1–996) fused to YFP was transiently co-expressed in N. benthamiana leaves with HA-tagged AVR-Pia, AVR-PikD, or PWL2 (without signal peptides). Proteins were extracted after 48 h and tagged proteins were detected in the extract (input) and after immunoprecipitation with anti-GFP beads (IP-GFP) by immunoblotting with anti-HA (α-HA) and anti-GFP (α-GFP) antibodies. Protein loading in the input is shown by Ponceau staining of the large RuBisCO subunit. The HMA domains of Pikp-1 and RGA5 were used as controls as well as the unrelated PWL2 effector of M. oryzae. The experiment was carried out twice with identical results.
