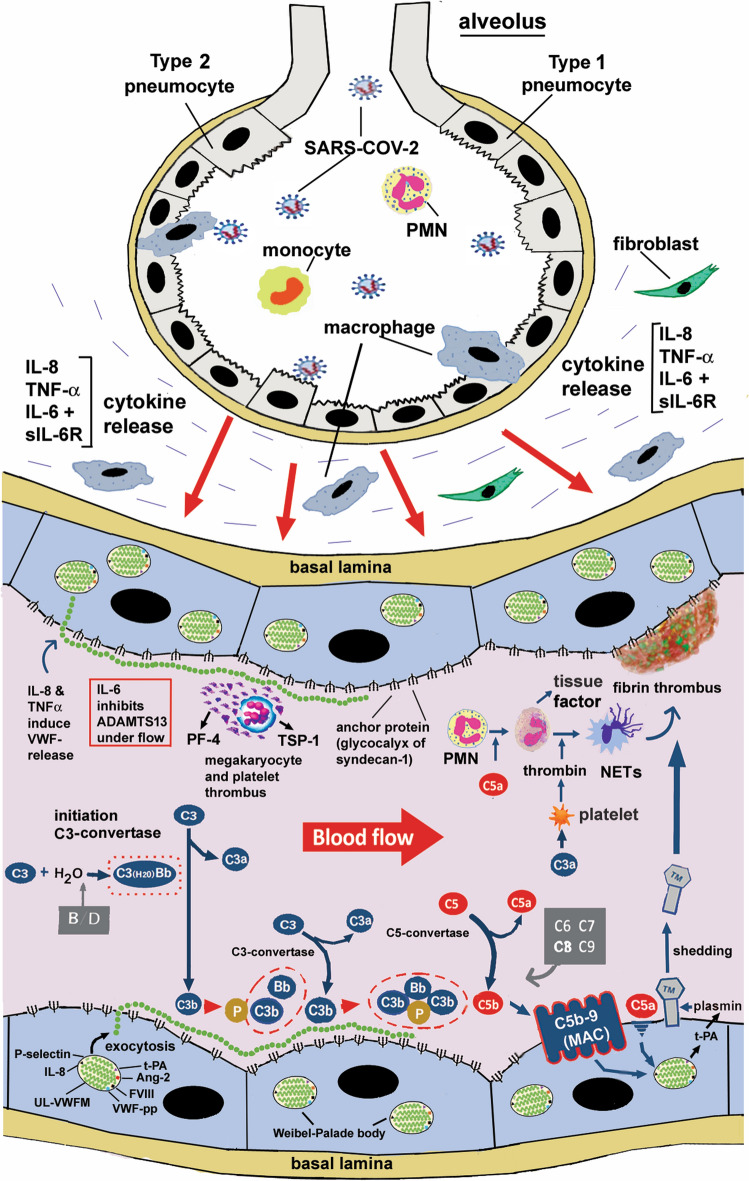
Fig. 3.
The role of UL-VWFMs in activation of the alternative complement pathway (AP) in the microvasculatures during COVID-19 thrombosis. SARS-CoV-2 invades lung alveoli from the respiratory tract and infects type 2 alveolar pneumocytes and macrophages. This causes release of cytokines from resident cells, such as macrophages, CD4-T lymphocytes and neutrophils. Inflammatory cytokines further stimulate release of cytokines from blood cells and vascular ECs, generating a cytokine storm. Consequently, IL-8, TNFα, and a complex of IL-6 and its soluble receptor (sIL-6R) stimulate vascular ECs, and induce exocytosis of UL-VWFMs from Weibel-Palade Bodies (WPBs). Under a high shear flow, UL-VWFMs undergo a conformational change, allowing ADAMTS13 more accessibility; however, IL-6 interferes with this interaction, resulting in inhibition of ADAMTS13 activity. In such microenvironments, the A1-loop domain of VWF binds platelets, forming platelet aggregates with or without involving resident megakaryocytes. The activated platelets release PF4 and TSP-1 from the α-granules, both of which bind to the A2-domain of VWF and block cleavage by ADAMTS13. The A1-loop domain of VWF binds to the heparan sulfate of syndecan-1 on the vascular EC surface, while the A2 domain binds to C3b generated by the AP activation. C3b bound to UL-VWFM anchored on the EC surface binds factor B, which is proteolyzed by factor D. Then binding of properdin to the C3b moiety as a stabilizer results in the formation of C3-convertase in the AP. Subsequent activation through the AP (C5-convertase) (C3bBbC3b) produces C5b, to which C6 ~ C9 bind, finally forming C5b-9 (MAC), which in turn activates endothelial cells (EC) together with endotheliopathy, UL-VWFM is a major constituent of WPBs, which also contain IL-8, Ang-2, t-PA, etc. The secretion of IL-8 into the circulation enhances UL-VWFM release and accelerates C3b binding to UL-VWFM on the vascular EC surface, promoting platelet thrombi formation. The released t-PA generates plasmin under microenvironments in which thrombomodulin (TM) on the vascular EC surface undergoes shedding. TM binds to thrombin to form a complex that inhibits thrombin activity, but activates protein C and thrombin-activatable fibrinolysis inhibitor (TAFI) to TAFIa (carboxypeptidase), which can proteolytically inactivate both C3a and C5a. Shedding of TM loses the antithrombotic function of vascular ECs, turning them into the thrombogenic surface