Figure 3.
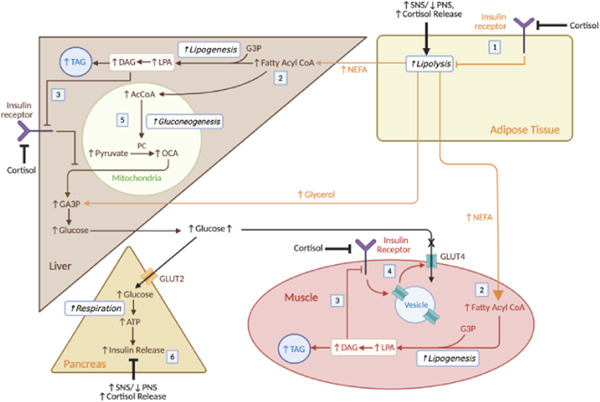
Physiological responses to sleep deprivation and impact on carbohydrate metabolism. (1) SNS dominance and cortisol drive lipolysis. The elevated cortisol levels reduce insulin sensitivity, reducing inhibition of lipolysis. (2) Lipolysis releases NEFA, which is converted into fatty acyl CoA to produce TAG in the muscle and liver. The build‐up of ectopic fat in the liver and muscle may cause other complications such as nonalcoholic fatty acid liver disease—also linked to sleep deprivation. 69 (3) When synthesising TAG, DAG is produced which activates PKCε in hepatocytes or PKCε/PKCθ in myocytes to inhibit insulin signalling. This effect is compounded by the cortisol‐induced reduction in insulin sensitivity at these cells. (4) This reduction in insulin sensitivity in the myocytes reduces GLUT4 insertion and hence glucose uptake in muscle, increasing glucose levels. (5) In the liver, the reduced insulin sensitivity and increased levels of AcCoA drive gluconeogenesis. (6) Normally, elevated glucose drives insulin release in the pancreas, but the relative increase in SNS activity and cortisol inhibit this response. Therefore, over long periods of time, glucolipotoxicity impairs β‐cell function. Though this is not shown, the autonomic imbalance and cortisol can also directly drive inverse processes to insulin (e.g., increasing hepatic gluconeogenesis, reducing glucose uptake via GLUT4 at adipose tissue and muscle), also contributing to T2DM. PNS, parasympathetic system; SNS, sympathetic nervous system; T2DM, type 2 diabetes mellitus [Color figure can be viewed at wileyonlinelibrary.com]
