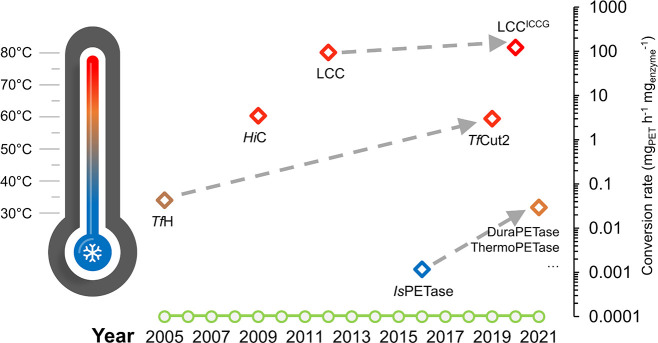
Figure 1.
Selected milestones of a 16-year-long history of identifying and engineering PET hydrolases. Both optimal reaction temperatures (in varying colors) for PET degradation and normalized maximal conversion rates (diamonds corresponding to logarithmic values on the vertical axis) calculated based on various publications are denoted, regardless of the material properties of applied PET substrates. The successes in raising the degradation performance using certain benchmark enzymes by both protein and process engineering are indicated by arrows with broken lines. TfH: hydrolase from T. fusca DSM43793;31HiC: Humicola insolens cutinase;42 LCC: leaf-branch compost cutinase;33,52IsPETase: I. sakaiensis PET hydrolase;16,34TfCut2: T. fusca KW3 cutinase;53 LCCICCG: LCC variant with indicated quadruple substitutions;16 DuraPETase, ThermoPETase, etc.: various thermostabilized IsPETase variants.54−56