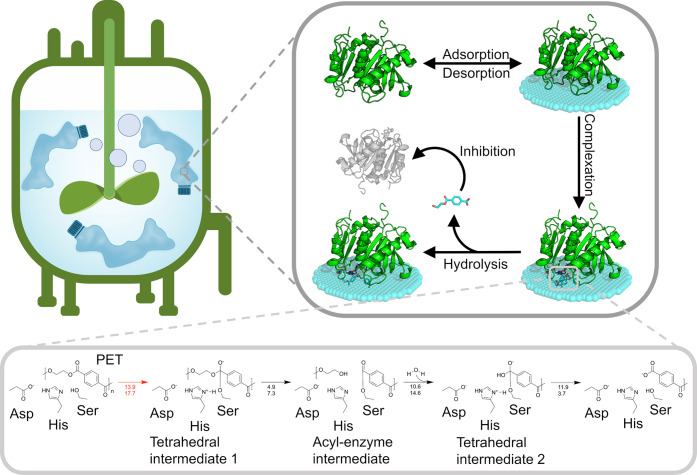
Figure 3.
Interfacial biocatalytic hydrolysis of PET and its reaction mechanism. The states of a PET hydrolase are schematically illustrated in the upper right panel. In the lower panel, individual steps of the hydrolysis reaction are schematically shown in line with their activation free-energy barriers in kcal·mol–1 summarized based on different studies.107,108 The reaction is initiated by a nucleophilic attack by a catalytic serine resulting in a tetrahedral intermediate stabilized by a catalytic histidine, an aspartic acid, and the oxyanion hole, followed by breakdown of the tetrahedral intermediate 1 into an acyl–enzyme intermediate and release of an alcohol. The aspartate–histidine pair activates the water for attack on the acyl–enzyme intermediate carbonyl, resulting in the formation of the second tetrahedral intermediate. The deacylation of this tetrahedral intermediate releases the carboxylic acid product. The rate-limiting step is regarded as the initial nucleophilic attack and highlighted in red with two free-energy activation barriers denoted. The top number is the Boltzmann-weighted average from 20 QM/MM MD simulations,108 and the bottom number comes from adiabatic mapping studies.107