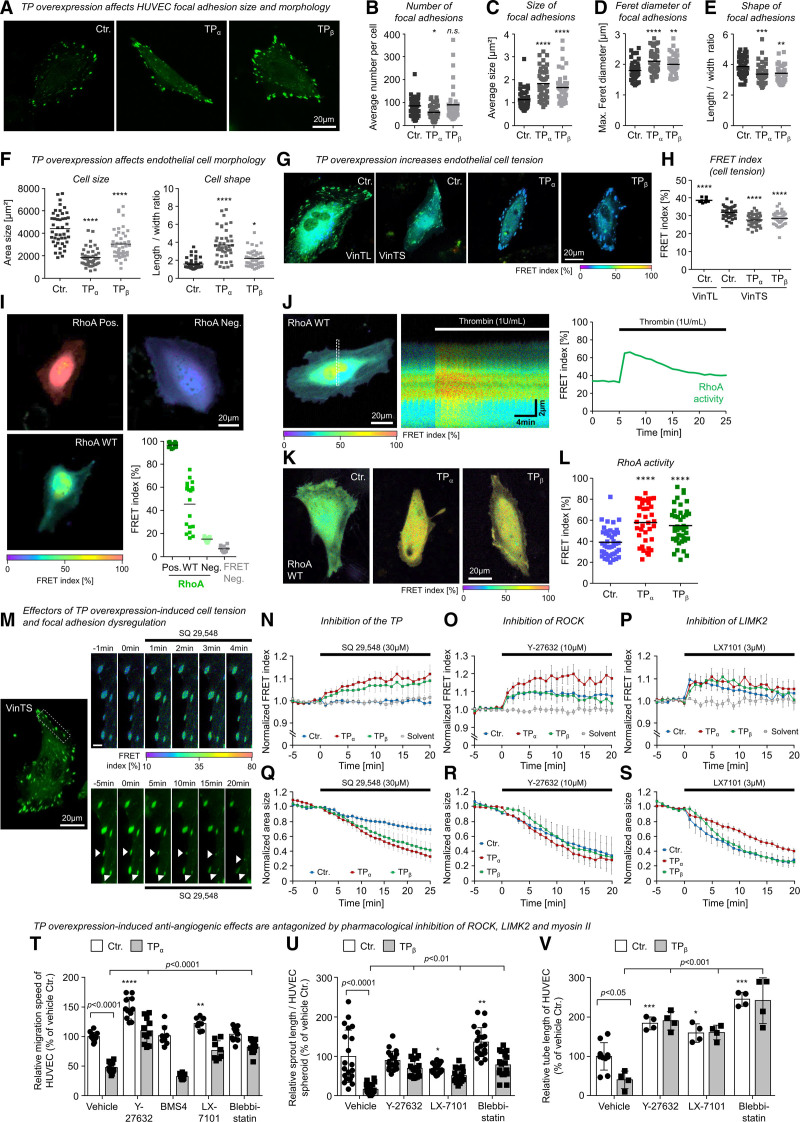
Figure 4.
The TP (thromboxane A2 receptor) induces morphological changes, cellular tension, and inhibition of angiogenesis-associated endothelial cell functions via a ROCK (Rho-associated coiled-coil containing protein kinase)-, LIMK2 (LIM domain kinase 2)-, and myosin II–dependent signal transduction pathway. A, Representative microscopic pictures of living control-transduced or TP-overexpressing human umbilical vein endothelial cells (HUVECs) in 2-dimensional culture additionally expressing the fluorescent vinculin-based tension biosensor (VinTS) for visualization of endothelial focal adhesions. B–E, TP overexpression reduces vascular endothelial cell size, induces an elongated cell shape (F), and favors the formation of large, irregularly shaped focal adhesions in particular at the cell margin of HUVECs (C–E). Statistical analysis of endothelial cell morphology parameters (n=43–45; *P<0.05/**P<0.01/***P<0.001/****P<0.0001 vs control-transduced HUVECs). G and H, TP overexpression increases vascular endothelial cell tension as indicated by a reduction of VinTS-associated Förster resonance energy transfer (FRET) in TP-overexpressing as compared with control-transduced HUVECs. In contrast, control HUVECs that express VinTL—a tailless tension-insensitive variant of the sensor—show a significantly higher FRET at focal adhesions as compared with control HUVECs expressing VinTS (n=9–45; ****P<0.0001 vs control-transduced HUVECs). I, Dynamic range of RhoA (Ras homolog gene family, member A) activity related FRET index of constitutive active (Pos.), wild-type, and dominant negative (Neg.) variants of the RhoA FRET biosensor. FRET Neg. represents separated donor/acceptor pair (mCerulean3/mVenus). J, Stimulation of HUVECs with 1 U/mL thrombin induces a transient activation of RhoA measured by the FRET biosensor. K and L, HUVECs transduced with lentiviral particles (Ctr., TPα, TPβ) were transfected with RhoA FRET biosensor. FRET index of TP-overexpressing cells was significantly higher than in Ctr. indicating a higher basal RhoA activity in these cells (Ctr., n=43; TPα, n=37; TPβ, n=42; ****P<0.0001). M, Representative microscopic pictures of a TPβ-overexpressing endothelial cell transiently expressing the VinTS biosensor treated with TP antagonist SQ-29548. Top shows an increasing FRET index within minutes after addition of SQ-29548, whereas bottom visualizes the SQ-29548–related regression of focal adhesions. Scale bar, 3 µm. N, Pharmacological inhibition of the TP using SQ-29548 (3×10−5 mol/L) reduces vascular endothelial cell tension in TP-overexpressing but not in control-transduced HUVECs. Q, SQ-29548 also induces a rapid regression of focal adhesion size in TP-overexpressing and control HUVECs. Similar effects on cell tension and regression of focal adhesions could be observed by inhibition of actomyosin regulators ROCK (O and R; 10 µM Y-27632) and LIMK2 (P and S; 3 µM LX-7101). Data are shown as mean±SEM (n=6–10). O–Q, Pharmacological inhibition of ROCK (Y-27632; 10 µmol/L), LIMK2 (LX-7101; 3 µmol/L), and myosin II (blebbistatin; 30 µmol/L) but not LIMK1 (BMS4; 0.5 µmol/L) significantly attenuates the inhibitory effect of TPα or TPβ overexpression on HUVEC migration (T; n=8–12), spheroid sprouting (U; n=17–20), and tube formation (V; n=4–10). Control-transduced HUVECs (Ctr.) served as appropriate comparative group. *P<0.05/**P<0.01/***P<0.001/****P<0.0001 vs vehicle-treated Ctr. All statistical analyses were performed using 1-way ANOVA followed by the Sidak multiple comparisons test.