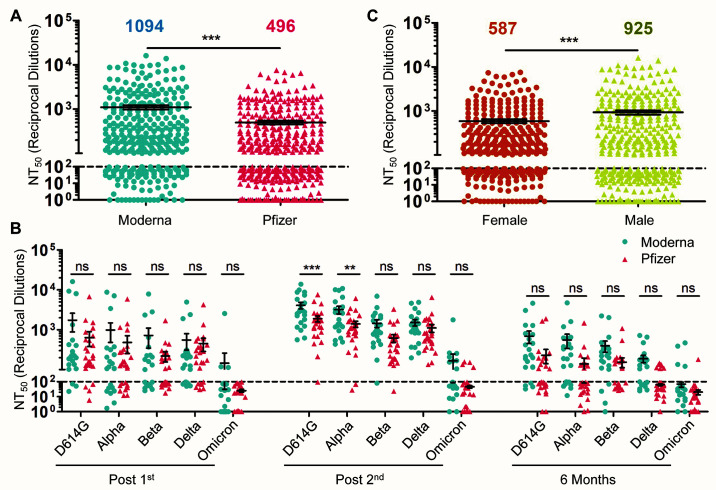
Fig. 3. nAb response is influenced by vaccine type and sex.
(A) HCWs were divided by types of mRNA vaccine received, either Moderna mRNA-1273 (n = 22) or Pfizer/BioNTech BNT162b2 (n = 26), and NT50 values were combined for all variants at all time points. Significance between vaccine type was determined by unpaired two-tailed t test with Welch’s correction and means are indicated at the top of the plot. (B) NT50 values of serum samples from individuals vaccinated with Moderna mRNA-1273 (n = 22) or Pfizer/BioNTech BNT162b2 (n = 26) were grouped by variant and time point, with significance determined by two-way repeated-measures ANOVA with Bonferroni’s correction. (C) NT50 values against all variants at post-first dose, post-second dose, and six months post-second dose combined were compared for male (n = 390) and female (n = 330) participants; mean NT50 values are displayed at the top of the plot, with significance determined by unpaired, two-tailed t test with Welch’s correction. For all panels, error bars indicate means ± standard errors, and the dashed horizontal line indicates the limit of detection (NT50 < 100). In all cases, *p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant.